What is Migrasome?
1 Introduction
The migrasome is a newly discovered organelle produced by migrating cells. When cells migrate, they leave filamentous structures called retraction fibers (RFs), and vesicles formed at the tips and intersections of RFs are defined as migrasomes. The sizes of these structures range from 0.5 to 3 μm. The cell contents can be transported to the migrasomes through RFs. Migrasomes can be released into the extracellular space through migration. When cells migrate and contract RFs, migrasomes remain in their original position and fall off from the RFs, eventually mediating the release of cytoplasmic contents and cell-to-cell communication. In addition, the exfoliation of RFs will produce another new type of small extracellular vesicle structure, called retractosome. Its physical location and origin are closely related to the migration. Migrasomes are not only involved in embryonic development, but also play an important role in other biological processes involving migrating cells, including immune response, angiogenesis, tissue regeneration and tumor metastasis.
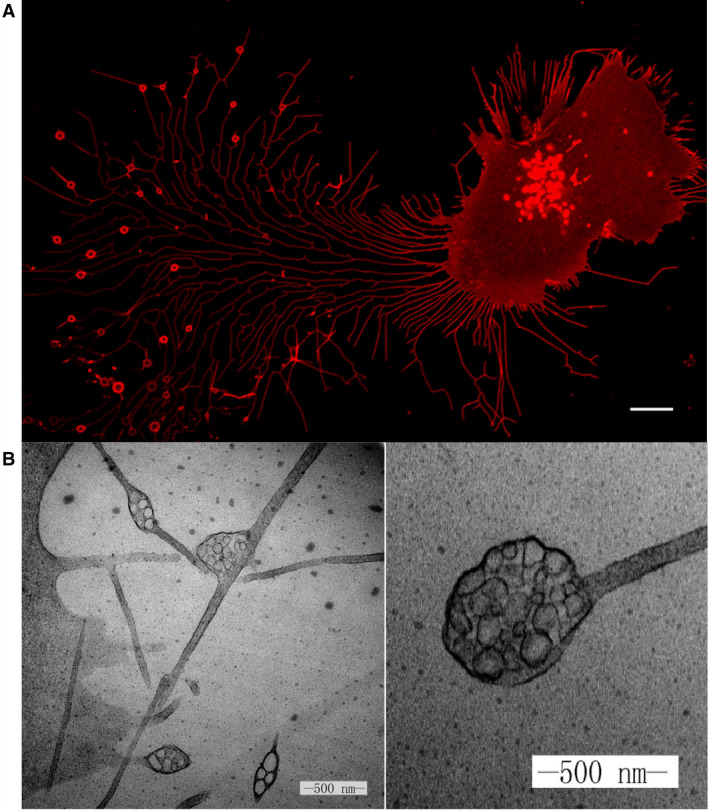 Fig. 1 Migrasomes from L929 cells.1,3
Fig. 1 Migrasomes from L929 cells.1,3
2 Discovery of Migrasomes and Retractosomes
Through transmission electron microscopy (TEM), scientists observed vesicle-like structures resembling open pomegranates appearing on the periphery of the cells. They tentatively named these structures "pomegranate-like structures (PLSs)" and published a study on PLSs in Cell Research in 2014. Yu et al. isolated and purified PLS by density gradient centrifugation and confirmed its identity by TEM. Further mass spectrometric analysis identified the tetraspanin TSPAN4 as a marker of PLS, and this was verified by constructing a plasmid fused with GFP protein. TSPAN4 is located in the cell membrane and retraction fibers (RFs) protruding from the surface of the cell membrane. And PLSs are formed at the tip or bifurcation of RF, and RF retracts and breaks to release PLSs into the medium. Before breaking up and disappearing, the average life cycle of PLSs is about 200-400 minutes. Because the formation of PLSs is closely related to the process of cell migration, they are officially named "migrasomes".
After cell migration, a large number of RFs are left behind and finally separated from the cells. TSPAN4 is uniformly distributed along RFs and appears as small dots along the path occupied by RFs in the process of RF separation from cells. In addition, scanning electron microscopy showed that there were a large number of small and round extracellular vesicles at the distal end of RFs. These small extracellular vesicles, called retractosomes, are much smaller than migrasomes, with diameters ranging from 50 nm to 250 nm. Retractosomes are thought to be produced by RFs broken during cell migration, but their specific physiological function has not been determined.
3 The Formation of Migrasomes
3.1 The cell migration model regulates the formation of migrasomes
Cell migration is a multi-step process in which cells constantly change their shape and polarity. In order to initiate migration, cells must acquire spatial asymmetry, resulting in a significant difference between the anterior and posterior poles. Extension of the active membrane occurs primarily at the anterior pole of the cell, leading to the formation of extended protrusions in the direction of movement, initiating cell migration, and stabilizing the process through interaction with the surrounding environment. The formation of migrasomes is closely related to cell migration because it occurs on residual bodies (RFs) left behind during migration. Studies have shown that the direction and speed of cell migration can affect the formation of migrasomes. Compared with cells with continuous migration on a straight track, the change in the direction of cell migration led to a decrease in the formation of migrasomes. In addition, there was a positive correlation between migration speed and migrasome formation. With the increase in speed of cell migration, the length of RFs increases, which will promote the formation of migrasomes.
3.2 Pairing of integrin and extracellular matrix (ECM) determines the formation of migrasomes
The formation of migrasomes depends on cell migration, which in turn depends on ECM adhesion. Cell migration involves the protruding of the front end of the cell, the formation of adhesion, and the retraction of the cell tail. Integrin is a transmembrane receptor that mediates cell adhesion to the extracellular matrix (ECM) and exists in the form of αβ heterodimer with large extracellular structural domains. The extracellular domain of integrin determines binding specificity and recognizes a variety of matrix ligands. Different integrins bind and adhere to different ECM proteins. The formation of migrasomes may require a specific pairing of integrin and its extracellular matrix (ECM) chaperone proteins. These proteins show different distribution patterns in organisms. Mechanistically, when cells migrate, integrins make cell migration possible. The pairing of integrin with ECM chaperone proteins can provide adhesion to retracted fibers. RFs are gradually produced after cell migration and finally form migrasomes. Studies have shown that pairing integrins with specific ECM chaperone proteins to obtain correct adhesion is the decisive factor for the formation of migrasomes. At the same time, the enrichment of integrins in migrasomes also provides specificity for entering specific receptor cells, which may be able to answer the questions of "where do migratory bodies come from" and "where do they go".
3.3 Key role of TSPAN4 in migrasome formation
TSPAN4 is the most abundant protein in migrasomes and was originally used as a marker to identify them. However, the role of TSPAN4 goes beyond its recognition as a protein marker. In fact, among 33 known mammalian tetraspanins, 14 kinds of TSPAN overexpression have been proven to promote the formation of migrasomes, and TSPAN4 is one of the most effective tetraspanins. TSPAN4 is recruited into migrasomes during the formation of migrasomes. Once recruited, TSPAN4 cannot migrate out and return RFs (the mechanism by which TSPAN4 does not return is not clear), suggesting that TSPAN4 may drive migrasome formation.
4 Basic Differences between Migrasomes, Retractosomes, and Exosomes
Migrasomes exhibit significant differences from exosomes, microvesicles, and apoptotic bodies in terms of size, life cycle, release mechanism, and molecular composition. Exosomes are small extracellular vesicles (sEVs) surrounded by a lipid bilayer membrane that are secreted by various types of cells and are quite different from migrasomes. The migrasome membrane is characterized by the presence of TSPAN4/7 and integrins α1, α3, α5, and β1, which are key structural markers. In addition, migrasomes are rich in proteins such as NDST1, PIGK, CPQ, and EOGT, but these proteins do not exist or are rarely detected in exosomes and can be used as unique protein markers of migrasomes. Retractosomes were significantly similar to migrasomes in protein composition but different from exosomes. Migrasomes are large vesicular structures distributed at the bifurcation point or end of RFs, while retractosomes is a small dot arranged in a beaded pattern. It can be distinguished from migrasomes according to its shape and location. However, migrasomes and retractosomes share markers such as PIGK, EOGT, and PCCA, although these markers are less enriched in retractosomes than migrasomes.
5 Distribution of Migrasomes
It is reported that migrasomes are widely distributed in various in vitro cultured cells and different species, including human, mouse, rat, zebrafish and chicken. The number, size, and length of RFs vary among cell types. Migrasomes are also found in a variety of tissues and body fluids, including ischemic brain tissue, platelets, blood, urine, intestines, eyes, placenta, microglia, neutrophils, macrophages, lungs, kidneys, the endoderm, the yolk syncytial layer of zebrafish embryos, and the middle layer of chorioallantoic membrane of chicken embryos. Although migrasomes are widely distributed, their exact role in human physiological or pathological conditions is still unclear. Further research is needed to fully understand the function and significance of migrasomes.
6 Biological Function of Migrasomes
Migrasomes can exert their biological functions through three different modes of action. First, the migrasomes act as packets of information that can be transmitted to a spatially defined location to send a signal to the surrounding cells. Secondly, the migrasomes act as a garbage disposal mechanism by which damaged organelles are expelled from the cells. Finally, the migrasomes play a role in mediating the lateral or horizontal transfer of RNA and protein. Migrasomes are not only involved in embryonic development, but also play an important role in other biological processes involving migrating cells, including immune response, angiogenesis, tissue regeneration, and tumor metastasis.
The most important function of migrasomes is probably their ability to serve as packets of information with a delivery address. Migrasomes are rich in signal molecules such as chemokines, cytokines, and growth factors. Mature migrasomes can release signal molecules through rupture or leakage, and then signal molecules can act on surrounding cells by binding to related receptors, which would activate signal cascades and change the state and behavior of these cells. Migrasomes can remain in the migration path of migrating cells, or once they are formed, they can be delivered to a space-limited location. Either way, migrasomes can act as a source of signal ligands at specific locations after cell migration for a long time. Therefore, migrasomes can integrate spatial and biological information, which is necessary for biological processes involving the coordinated action of different migrating cells. In addition, this "latent" effect released by ligands may provide another opportunity to fine-tune ligand-mediated intercellular communication. In addition to integrating spatial and biological information, migrasomes can also deliver combinatorial signals by encapsulating multiple signaling molecules, thus becoming the perfect carrier for combinatorial signals.
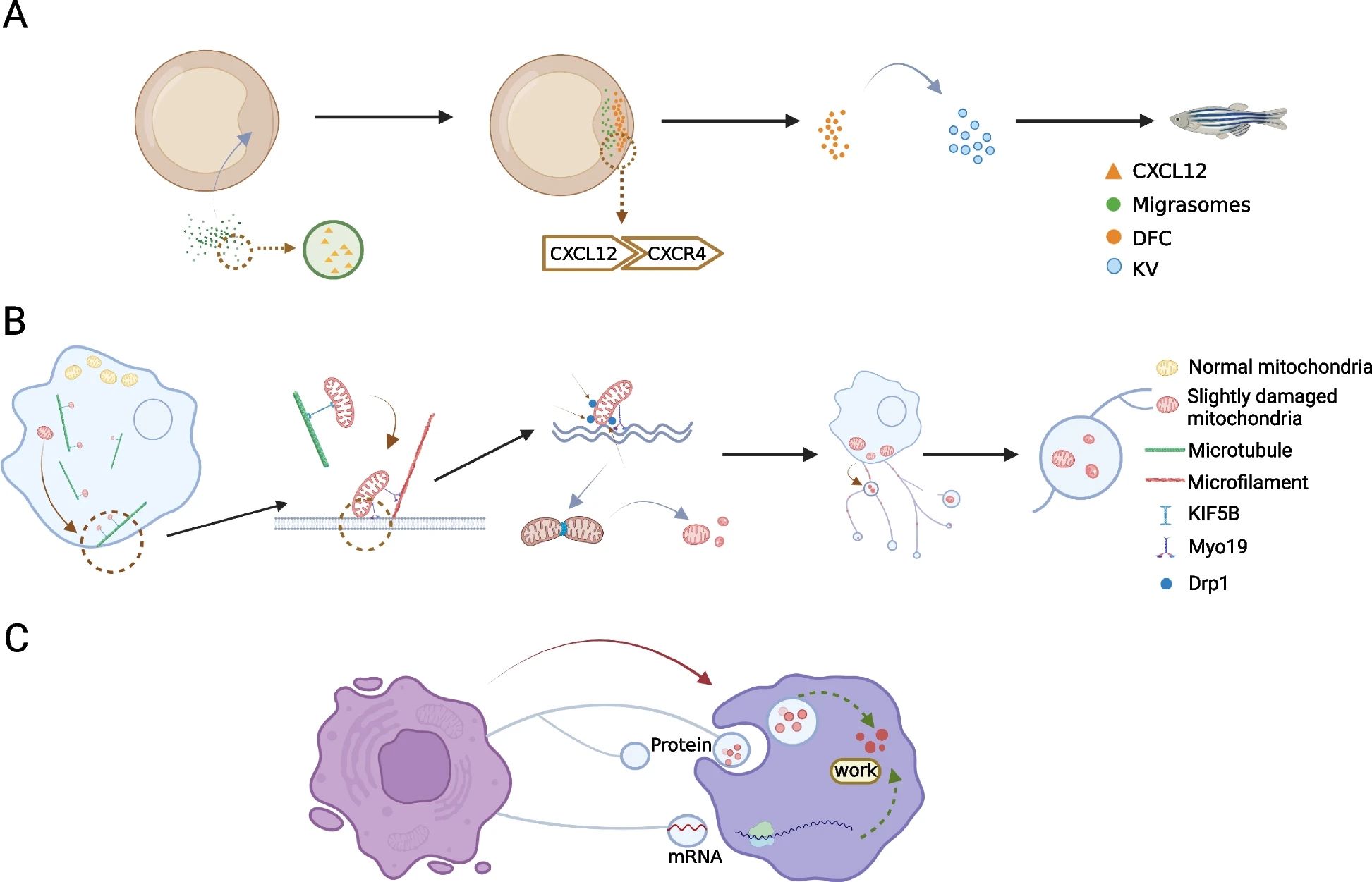 Fig. 2 Migrasomes mediate cell-to-cell communication.2,3
Fig. 2 Migrasomes mediate cell-to-cell communication.2,3
The second mode of action is to deal with unwanted cellular contents. A recent study found that when migrating cells are subjected to mild mitochondrial stress, they can treat damaged mitochondria through migrasomes, a process known as mitocytosis. In mitochondrial heterogeneous cell experiments, migrasomes isolated from cells containing normal and mutated mitochondrial DNA (mtDNA) show that most of the mtDNA in migrasomes is in mutant form. When exposed to mild mitochondrial stress, damaged mitochondria are selectively transported to migrasomes, indicating that migrasomes selectively transport impaired mitochondria, indicating that this is a highly regulated process. Mitocytosis and mitophagy are important mechanisms for maintaining mitochondrial homeostasis in migrating cells. Mild mitochondrial stress can cause mitocytosis, while mitophagy can be activated under severe damage caused by pathological conditions. Studies have shown that these two processes act as different systems to respond to different levels of mitochondrial stress, and both are essential for maintaining mitochondrial homeostasis. Understanding their role may lead to new treatments for diseases related to mitochondrial dysfunction.
The third mode of action is the lateral or horizontal transfer of cellular contents, including mRNAs and proteins. In cells cultured in vitro, scientists can often observe that the migrasomes produced by one cell can be swallowed by the surrounding cells. In addition, cellular contents such as proteins and vesicles can be observed in migrasomes, indicating that migrasomes can mediate the lateral transfer of substances between cells. Recently, researchers have found that migrasomes contain RNAs. The sequencing of the RNAs in migrasomes shows that most of the RNAs in migrasomes are full-length mRNAs with translation ability. Compared with RNAs in parent cells, migrasomes contain a high abundance of mRNA subsets, including Pten mRNA. When migrasomes containing Pten mRNA were added into tumor cell lines with Pten deficiency, the Pten mRNA was translated in tumor cells, which almost completely eliminated the P-Akt signal and inhibited the proliferation of these cells. It indicates that the lateral transfer of mRNAs can modify the receptor cells.
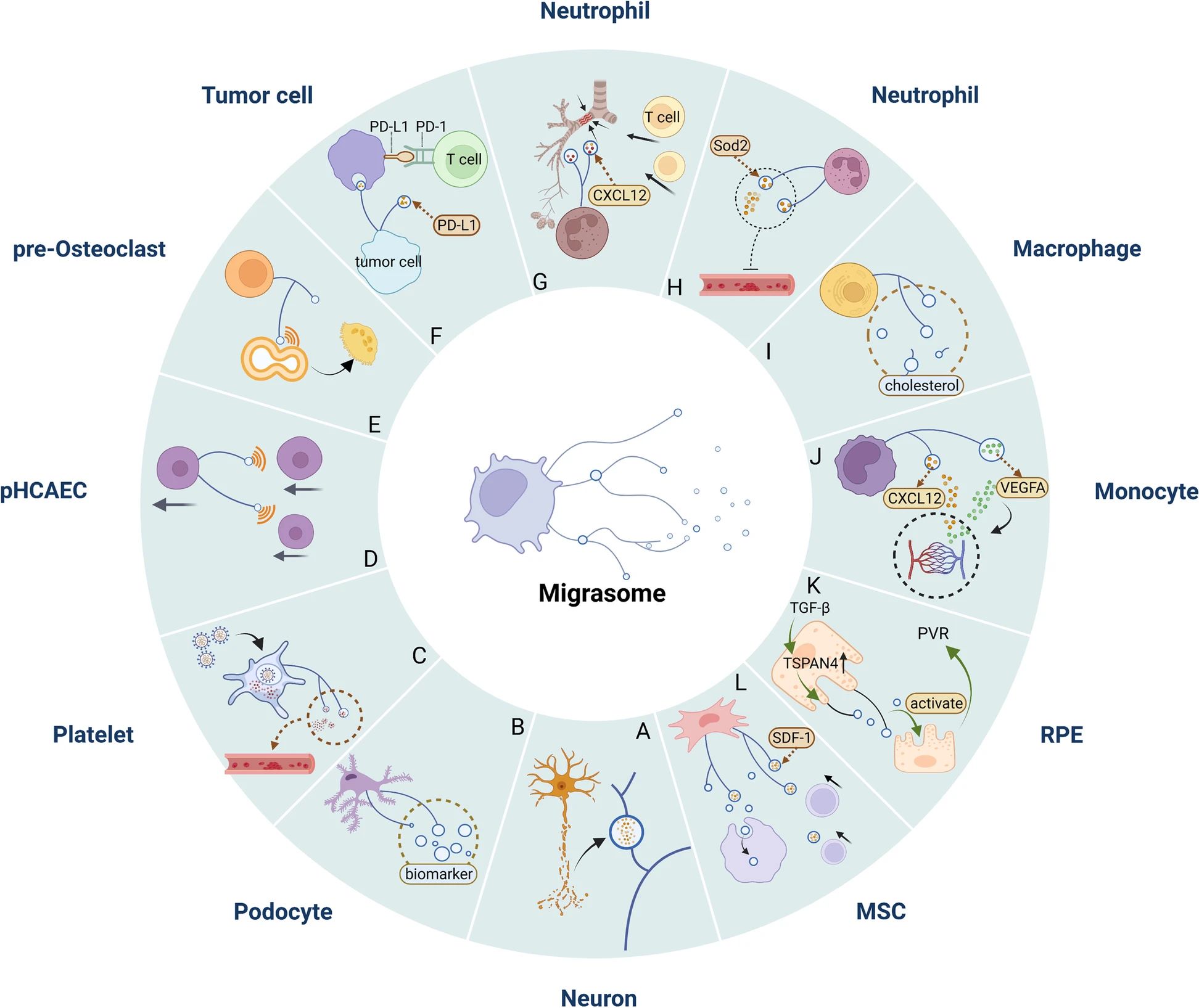 Fig. 3 Migrasomes mediate the pathological process of diseases.2,3
Fig. 3 Migrasomes mediate the pathological process of diseases.2,3
Cell migration is a basic phenomenon and mechanism for regulating dynamic balance in vivo. It is associated with normal cellular physiology and human diseases, including embryogenesis, trauma healing, immune defense, cardiovascular disease, eye disease, tumor biology, osteoporosis, diabetes, nephropathy, and chronic inflammatory diseases such as rheumatoid arthritis and multiple sclerosis. In fact, migrasomes are intrinsically related to cell migration. But so far, the research on the role of migrasomes in diseases is still in its infancy. Therefore, it is necessary to explore the specific molecular mechanism of their biogenesis and regulation as soon as possible. In recent years, the physiological and pathological functions of migrasomes or its related events have been studied in zebrafish developmental models, mouse models of mild mitochondrial stress in neutrophils and macrophages, mouse models of ischemic stroke, pan-induced mouse models of nephropathy, and in vitro cancer cell proliferation. Due to the ubiquity of cell migration in homeostasis and disease regulation, it is speculated that the function of migrasomes goes beyond the above. In the future, more basic and clinical studies are needed to further study the role of migrasomes in physiological and pathological processes.
Related Products
At Creative Biolabs, we prioritize your scientific success and are here to help if you are working to find a suitable antibody for your migrasome research and diagnosis.
| Targets | Clone | Applications | Species Reactivity | Cat. |
| TSPAN7 | SN1a | FC | Human | CBMAB-C2067-CQ |
| TSPAN7 | SN1a M3-3D9 | FC | Human | CBMAB-C12620-LY |
| TSPAN7 | CBYJT-5084 | IHC, IHC-P | Human | CBMAB-T4662-YJ |
| TSPAN7 | CBYJT-5081 | ELISA | Human | CBMAB-T4659-YJ |
| TSPAN7 | CL0262 | IHC, IHC-P, WB | Human, Mouse | CBMAB-1667-YC |
| GFP | 11F8-G2 | WB | Human | CBMAB-A3392-LY |
| GFP | CBLG1-979 | ELISA, IHC, WB | Jellyfish | CBMAB-G3126-LY |
| GFP | CBLG1-977 | ELISA, IP | Jellyfish | CBMAB-G3124-LY |
| GFP | CBLG1-976 | ELISA, IF, IP, WB | Jellyfish | CBMAB-G3123-LY |
| GFP | CBLG1-975 | ELISA, IP, WB | Jellyfish | CBMAB-G3122-LY |
| NDST1 | 2F11 | ELISA, WB | Human | CBMAB-N1592-WJ |
| NDST1 | CBWJN-0076 | ELISA, IHC, WB | Human | CBMAB-N1591-WJ |
| NDST1 | 1G10 | ELISA, IHC-P, WB | Human | CBMAB-N0212-WJ |
| CXCL12 | D8G6H | IHC-P | Human | CBMAB-CP2375-LY |
| CXCL12 | D32F9 | WB, IP | Human, Mouse, Rat | CBMAB-CP2374-LY |
| CXCL12 | 2B1 | sELISA, ELISA | Human | CBMAB-A2016-LY |
| CXCL12 | 1B2 | sELISA, ELISA | Human | CBMAB-A2013-LY |
| CXCL12 | W15149A | WB | Human, Mouse | CBMAB-C11359-LY |
| CXCR4 | CF126 | ELISA, WB, FC | Human, Mouse | CBMAB-FT347LY |
| CXCR4 | RM0366-30L4 | WB, IHC-F, Neutralization, FC | Mouse | CBMAB-C11413-LY |
| CXCR4 | MM0224-7L25 | IHC-F, Neutralization, FC | Human | CBMAB-C11412-LY |
| CXCR4 | D4Z7W | IHC-P, FC | Human | CBMAB-C3253-CN |
| CXCR4 | 5J286 | ELISA, FC, IHC | Human, Monkey | CBMAB-C3251-CN |
| DRP1 | D9A1 | WB, IF (ICC), FC | Human, Mouse, Rat | CBMAB-CP0508-LY |
| DRP1 | D3A4 | WB | Rat, Human, Mouse, Monkey | CBMAB-CP0507-LY |
References
- Yu, Shunbang, and Li Yu. "Migrasome biogenesis and functions." The FEBS Journal 289.22 (2022): 7246-7254.
- Jiang, Yuyun, et al. "Migrasomes, a new mode of intercellular communication." Cell Communication and Signaling 21.1 (2023): 105.
- Distributed under Open Access license CC BY 4.0, without modification.


