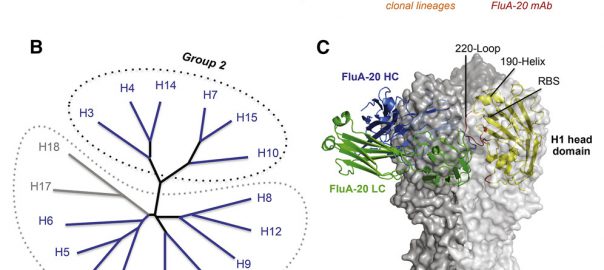
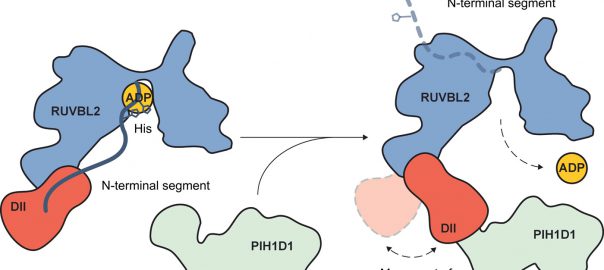
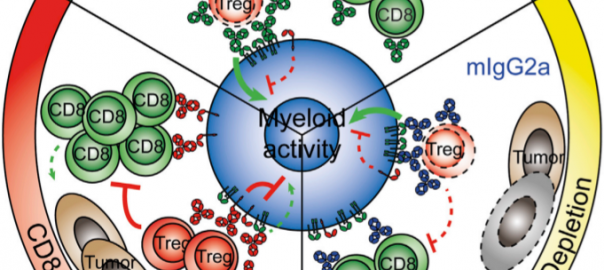
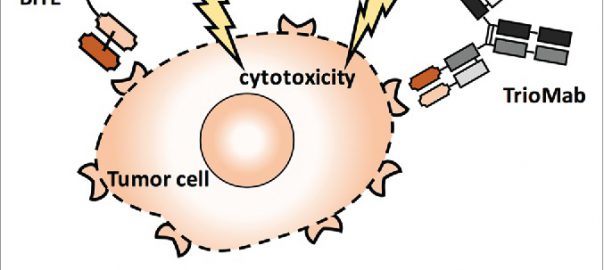
Cell: Scientists have Identified Protective Antibodies in Humans that are Expected to Help Develop New and Effective Antimalarial Vaccine
In a recent study published in the international journal Cell, scientists from Oxford University and other institutions identified a human antibody that could inhibit malaria parasites from entering blood cells. Related researchRead More…