Recently, the Mayo Clinic in the United States gave the following answera about COVID-19. 1.What is coronavirus? SARS-CoV-2 is a new type of coronavirus that researchers have recently discovered that it canRead More…
Development Status and Trend of Antibody Drugs for Ophthalmic Diseases in the World
Among the drugs for ophthalmic diseases, anti-VEGF drugs are developing rapidly, and aflibercept is amplified rapidly. At the same time, the development of new targets, new drug delivery methods and bispecific antibodiesRead More…
Cell: Scientists have Identified Protective Antibodies in Humans that are Expected to Help Develop New and Effective Antimalarial Vaccine
In a recent study published in the international journal Cell, scientists from Oxford University and other institutions identified a human antibody that could inhibit malaria parasites from entering blood cells. Related researchRead More…
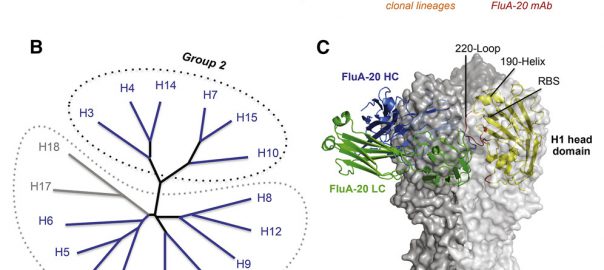
Cell: Human Antibody Reveals Potential Weakness of Influenza Virus
Scientists, from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, reported that the ever-changing “head” of the flu virus protein has an unexpected Achilles’ heel. TheRead More…
Science: Scientists Find Proteins that Mediate Key Life Activities of Cancer Cells
Protein is an integral part of life. In cells, proteins combine to form large macromolecular complexes that cooperate with each other to perform specific functions. A large number of cancer studies haveRead More…
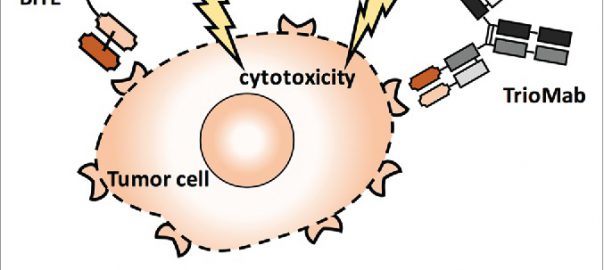
How to Use New Antibody Therapy to Treat a Variety of Human Diseases?
1. Nat Biotechnol: Edible antibody molecules contribute to the treatment of gastrointestinal diseases Therapeutic antibodies are increasingly used clinically to treat a variety of diseases. However, oral gut-targeted antibodies remain a challenge becauseRead More…
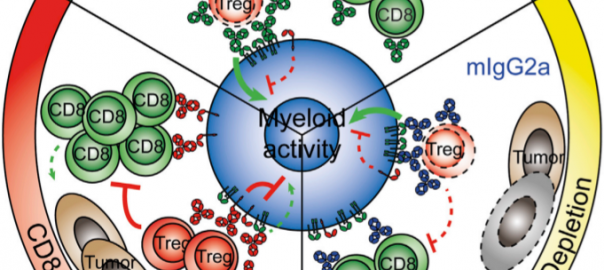
The Latest Development of PARP Inhibitors, Bispecific Antibodies and other Hot Spots
In 2018, Loxo Oncology’s “unlimited cancer” targeted therapy Vitrakvi was approved by FDA, becoming the first targeted therapy for patients with NTRK gene fusion variant solid tumors. Loxo, on the other hand,Read More…
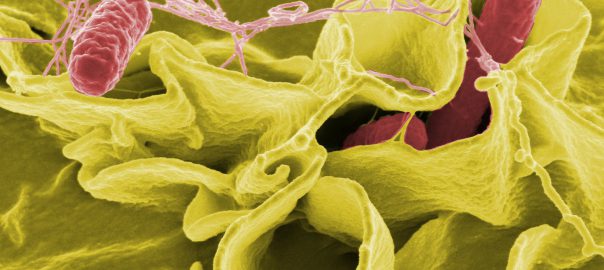
New Studies have Found that CD40 Molecules are the Key Entry Point for Dangerous Bacteria
A new study identifies a single molecule as a key entry point for two dangerous bacteria to break through cell barriers and cause disease. The findings, published March 19 in the journalRead More…
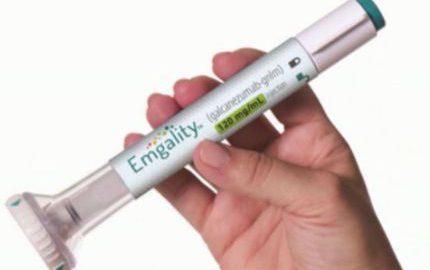
New Drug for Episodic Cluster Headache! Lilly Receives FDA Priority Review Designation for A Novel CGRP Antibody Emgality for the Preventive Treatment
Pharmaceutical giant and Company Eli Lilly Recently announced that the U.S. Food and Drug Administration (FDA) has granted Priority Review for its supplemental Biologics License Application (sBLA) for Emgality (galcanezumab-gnlm) injection forRead More…
New Drugs for Multiple Myeloma! Sanofi CD38 Targeted Antibody Therapy – Isatuximab’s First Phase III Clinical Study was Successful
French pharmaceutical giant Sanofi recently announced that the key phase III clinical study ICARIA-MM to evaluate isatuximab in the treatment of recurrent/refractory multiple myeloma (R/R MM) has reached the main end point. TheRead More…