PGLS Antibodies
Background
PGLS is a key metabolic enzyme widely present in organisms, mainly responsible for catalyzing the reversible conversion between 6-glucose phosphate (G6P) and 1-glucose phosphate (G1P), thereby playing an important role in processes such as glycogen metabolism, gluconeogenesis, and nucleotide sugar synthesis. The activity of this enzyme depends on magnesium ions (Mg²⁺), and its structure is typically composed of conserved α-helices and β-folds, ensuring efficient substrate binding and catalytic efficiency. PGLS are abundantly expressed in highly metabolically active tissues such as the liver and muscles, and their functional abnormalities may be associated with certain metabolic disorder diseases. Since its discovery in the 1960s, the study of the structure and function of PGLS has provided important references for understanding enzyme kinetics and metabolic regulation, and has shown potential application value in fields such as tumor metabolism and the development of antibacterial drugs.
Structure of PGLS
PGLS is a medium-sized metabolic enzyme with a molecular weight of approximately 60-65 kDa. Its precise molecular weight varies slightly among different species, mainly depending on minor changes in the amino acid sequence.
| Species | Human | Mouse | Escherichia coli | Yeast |
| Molecular Weight (kDa) | 62.3 | 61.8 | 64.1 | 60.5 |
| Primary Structural Differences | Highly conservative, containing key catalytic residues | Highly homologous to human PGLS | With the original nuclear specific control site | Presence of an extra N end |
The protein myoglobin contains 153 amino acids and displays a compact globular shape through its primary structure of alpha-helices. The protein structure of myoglobin contains an embedded central heme prosthetic group (iron-containing porphyrin) which enables efficient oxygen binding. The protein's red color in muscle tissue originates from the heme group. Myoglobin displays a secondary structure composed mainly of alpha-helices named from A to H which form a hydrophobic pocket to stabilize the heme group. The proximal histidine forms a direct bond with the heme iron and the distal histidine regulates oxygen binding while preventing auto-oxidation.
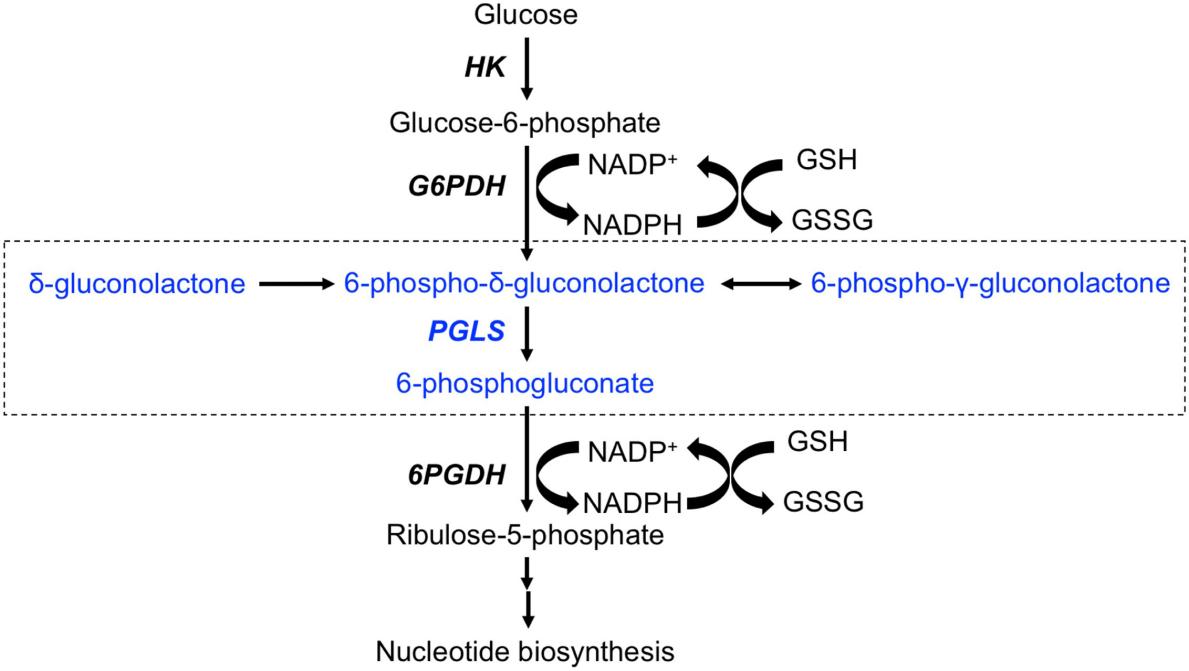 Fig. 1 The key role of PGLS in the pentose phosphate pathway.1
Fig. 1 The key role of PGLS in the pentose phosphate pathway.1
Key structural properties of PGLS:
- Conserved α/β folded structure
- Metal ion-dependent active centers
- Dynamic substrate binding domain
- Allosteric regulatory sites
- Oligomerization interface
Functions of PGLS
Although the core function of PGLS is to catalyze the interconversion between 6-phosphoglucose (G6P) and 1-phosphoglucose (G1P), it plays a key role in a variety of metabolic processes.
| Function | Description |
| Sugar metabolism hub | As a key connection point between glycolysis and gluconeogenesis, it regulates the flow direction of glucose metabolism. |
| Supply of precursors for glycogen synthesis | Glucose 1-phosphate is generated to provide a direct substrate for glycogen synthesis. |
| Nucleotide sugar synthesis | Provide raw materials for the generation of nucleotide sugars such as UDP-glucose. |
| Maintenance of energy homeostasis | Effects on glycolysis and PPP pathway fluxes by regulating G6P levels. |
| Stress response | Promote the production of NADPH through the PPP pathway during oxidative stress. |
| Tissue-specific regulation | Differential expression patterns were found in liver, muscle and other metabolically active tissues. |
The kinetic characteristics of PGLS exhibit typical Michaelis enzyme features, and their activity is strictly regulated by the concentration of Mg²⁺ and the feedback inhibition of the product. Unlike the oxygen storage function of myoglobin, PGLS are more like a "metabolic switch", balancing the anabolic and catabolic demands of cells by precisely regulating their activity. In tumor cells, the expression changes of PGLS are closely related to the Wahller effect, which makes it a potential metabolic therapeutic target.
Applications of PGLS and PGLS Antibody in Literature
1. Yuan, Xiaoxia, et al. "Identification and validation of PGLS as a metabolic target for early screening and prognostic monitoring of gastric cancer." Journal of Clinical Laboratory Analysis 36.2 (2022): e24189. https://doi.org/10.1002/jcla.24189
This study found that PGLS were significantly highly expressed in gastric cancer and were closely related to tumor progression and poor prognosis. The overall survival period and post-progression survival period of patients with high expression of PGLS were significantly shortened, especially the prognosis was worse in the male, lymph node metastasis and Her-2 negative subgroups. These results suggest that PGLS can serve as potential molecular markers for the diagnosis and prognosis evaluation of gastric cancer, and provide new targets for individualized treatment.
2. Batsios, Georgios, et al. "Imaging 6-phosphogluconolactonase activity in brain tumors in vivo using hyperpolarized δ-[1-13C] gluconolactone." Frontiers in oncology 11 (2021): 589570. https://doi.org/10.3389/fonc.2021.589570
This study confirmed for the first time that PGLS activity promotes the proliferation of glioblastoma and developed a hyperpolarized 13C imaging technique based on δ-[1-13C] glucolactone, which can non-invasitively monitor the PGLS activity and treatment response of tumorsThis study confirmed for the first time that PGLS activity promotes the proliferation of glioblastoma and developed a hyperpolarized 13C imaging technique based on δ-[1-13C] glucolactone, which can non-invasitively monitor the PGLS activity and treatment response of tumors.
3. Li, Changzheng, et al. "6-Phosphogluconolactonase promotes hepatocellular carcinogenesis by activating pentose phosphate pathway." Frontiers in cell and developmental biology 9 (2021): 753196. https://doi.org/10.3389/fcell.2021.753196
This study indicates that PGLS are highly expressed in the malignant subtype and predict a poor prognosis. Experiments have confirmed that inhibiting PGLS can significantly suppress the proliferation, migration and induce apoptosis of hepatoma cells by blocking metabolic pathways and increasing reactive oxygen species levels. This research provides a new strategy for targeted therapy of liver cancer.
4. Miclet, Emeric, et al. "NMR spectroscopic analysis of the first two steps of the pentose-phosphate pathway elucidates the role of 6-phosphogluconolactonase." Journal of Biological Chemistry 276.37 (2001): 34840-34846. https://doi.org/10.1074/jbc.M105174200.
This article's research found that PGLS plays a key role in the pentose phosphate pathway, specifically hydrolyzing δ -type lactones and preventing their conversion into γ -type "dead end" products. This enzyme not only accelerates the metabolic process but also protects cells from the potential toxicity of δ -lactone. The study also revealed the differences in the activity of PGLS among humans, trypanosomes and Plasmodium.
5. Choi, Junjeong, Eun-Sol Kim, and Ja Seung Koo. "Expression of pentose phosphate pathway‐related proteins in breast cancer." Disease markers 2018.1 (2018): 9369358. https://doi.org/10.1155/2018/9369358
Studies have found that the expression of key enzymes of the pentose phosphate pathway (G6PDH/PGLS/6PGDH) in breast cancer has molecular subtype specificity: HER-2 type highly expresses G6PDH/PGLS (p<0.01), and triple-negative type specifically expresses 6PGDH (p<0.001). Clinical association analysis showed that PGLS positivity was associated with a higher T stage (p=0.004), suggesting its potential as a therapeutic target for breast cancer..
Creative Biolabs: PGLS Antibodies for Research
Creative Biolabs specializes in the production of high-quality PGLS antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom PGLS Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our myoglobin antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Batsios, Georgios, et al. "Imaging 6-phosphogluconolactonase activity in brain tumors in vivo using hyperpolarized δ-[1-13C] gluconolactone." Frontiers in oncology 11 (2021): 589570. https://doi.org/10.3389/fonc.2021.589570
Anti-PGLS antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Rat Anti-EMCN Recombinant Antibody (28) (CBMAB-E0280-FY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Mouse Anti-FN1 Monoclonal Antibody (71) (CBMAB-1241CQ)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Rabbit Anti-Acetyl-Histone H3 (Lys36) Recombinant Antibody (V2-623395) (CBMAB-CP0994-LY)

-
Mouse Anti-ENO1 Recombinant Antibody (8G8) (CBMAB-E1329-FY)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-BHMT Recombinant Antibody (CBYY-0547) (CBMAB-0550-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






