AGK Antibodies
Background
AGK is a key metabolic enzyme, mainly present in the muscles, hearts and brain tissues of vertebrates. It catalyzes the reversible reaction between creatine and adenosine triphosphate (ATP), generating creatine phosphate and adenosine diphosphate (ADP), thereby rapidly providing energy reserves for cellular activities, especially playing a significant role during intense exercise or high energy consumption. AGK was first isolated in the mid-20th century. The study of its structure and function provides an important basis for the regulation of energy metabolism, muscle physiology and the diagnosis of related diseases (such as myocardial infarction). As a core molecule in metabolic pathways, the efficient reaction mechanism and tissue-specific distribution of AGK make it one of the research hotspots in biochemistry and clinical medicine.
Structure of AGK
AGK is a key metabolic enzyme with a molecular weight of approximately 24-26 kDa. Its specific size may vary slightly depending on the species and post-translational modifications (such as phosphorylation).
| Species | Human | Mouse | Rats | Bovine |
| Molecular Weight (kDa) | 25.5 | 25.3 | 25.4 | 25.6 |
| Primary Structural Differences | Conserved catalytic domain | Highly homologous and functionally similar | Highly similar to human AGK | Similar role in lipid metabolism |
AGK catalyzes the phosphorylation of acylglycerol (such as monoacylglycerol) through the conserved phosphorylation site of its active center, participating in lipid signal transduction and energy metabolism regulation. Its structural stability depends on the hydrophobic core and key disulfide bonds, ensuring that it still maintains functional activity under cellular stress conditions.
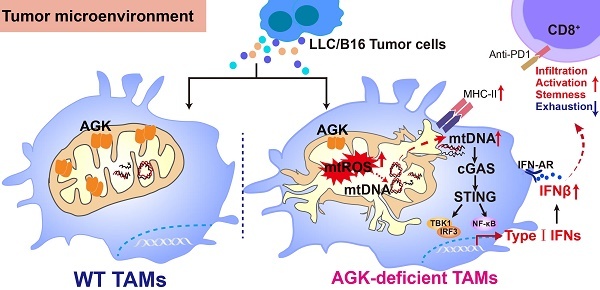 Fig. 1 AGK deficiency enhances the anti-tumor immune function of TAMs through the cGAS-STING-I interferon pathway.1
Fig. 1 AGK deficiency enhances the anti-tumor immune function of TAMs through the cGAS-STING-I interferon pathway.1
Key structural properties of AGK:
- Conserved kinase folding structure
- Hydrophobic substrate binding pockets
- ATP binding sites and key catalytic residues
- Regulatory structural components
Functions of AGK
AGK is a key metabolic enzyme, mainly involved in lipid metabolism and energy homeostasis regulation, and plays an important role in various physiological processes at the same time.
| Function | Description |
| Lipid phosphorylation | Catalyze the phosphorylation of monoacylglycerol (MAG) and diacylglycerol (DAG), generating lysophosphatidic acid (LPA) and phosphatidic acid (PA), and regulate lipid signaling. |
| Regulation of energy metabolism | By generating phosphatidic acid involved in three acylglycerol (TAG) synthesis and mitochondria beta oxidation, affecting cell energy supply. |
| Maintenance of mitochondrial function | Some subtypes are located in mitochondria, participate in cardiolipin metabolism, and affect the stability of mitochondrial membranes and the efficiency of electron transport chains. |
| Cell signal transduction | Its product LPA can act as a signaling molecule to activate G protein-coupled receptors (GPCRs), regulating cell proliferation, migration and inflammatory responses. |
| Disease association | Abnormal expression of AGK is associated with metabolic syndrome, cardiovascular diseases and certain cancers, and may become a potential therapeutic target. |
The enzymatic activity of AGK is regulated by ATP/ADP levels, phosphorylation modification and subcellular localization. Its substrate preferences (such as selectivity for MAG vs DAG) may vary in different tissues.
Applications of AGK and AGK Antibody in Literature
1. Huang, Shanshan, et al. "Up-regulated acylglycerol kinase (AGK) expression associates with gastric cancer progression through the formation of a novel YAP1-AGK–positive loop." Journal of Cellular and Molecular Medicine 24.19 (2020): 11133-11145. https://doi.org/10.1111/jcmm.15613
This article indicates that the research on AGK antibodies has found that AGK is highly expressed in gastric cancer and is associated with a poor prognosis. AGK promotes tumor proliferation and metastasis by activating the YAP1/TEAD signaling pathway. Meanwhile, YAP1 can trans-activate the expression of AGK, forming a positive feedback loop. AGK antibodies can be used as potential research tools.
2. Zhu, Qian, et al. "Acylglycerol kinase promotes tumour growth and metastasis via activating the PI3K/AKT/GSK3β signalling pathway in renal cell carcinoma." Journal of hematology & oncology 13 (2020): 1-16. https://doi.org/10.1186/s13045-019-0840-4
This article indicates that AGK, as a novel lipid kinase, may play a pro-cancer role in metastatic renal cell carcinoma. Its expression characteristics are related to the poor prognosis of patients and may become a new therapeutic target.
3. Du, Qiuyang, et al. "Acylglycerol kinase inhibits macrophage anti-tumor activity via limiting mtDNA release and cGAS-STING-type I IFN response." Theranostics 15.4 (2025): 1304. https://www.thno.org/v15p1304.htm
Studies have found that AGK deficiency in macrophages activates the cGAS-STING-I interferon pathway by inducing mitochondrial damage and mtDNA release, thereby enhancing the anti-tumor immunity of CD8+T cells (based on experimental evidence from the B16/LLC mouse model).
4. Sun, Fei, et al. "Acylglycerol kinase promotes ovarian cancer progression and regulates mitochondria function by interacting with ribosomal protein L39." Journal of Experimental & Clinical Cancer Research 41.1 (2022): 238. https://doi.org/10.1186/s13046-022-02448-5
Studies have found that AGK is highly expressed in ovarian cancer. It maintains mitochondrial function by binding to RPL39, promotes the characteristics of tumor stem cells and cisplatin resistance (based on clinical samples and in vivo and in vitro experimental evidence).
5. Zhang, Zhibo, et al. "Netupitant Inhibits the Proliferation of Breast Cancer Cells by Targeting AGK." Cancers 16.22 (2024): 3807. https://doi.org/10.3390/cancers16223807
Studies have found that the anti-tumor drug Netupitant inhibits the kinase activity of AGK by targeting it and blocks the PI3K/AKT/mTOR pathway, thereby suppressing the proliferation of breast cancer cells and inducing apoptosis (based on in vitro and nude mouse experimental evidence).
Creative Biolabs: AGK Antibodies for Research
Creative Biolabs specializes in the production of high-quality AGK antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Western Blot, Flow Cytometry, Immunohistochemistry, and other diagnostic methodologies.
- Custom AGK Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our AGK antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Du, Qiuyang, et al. "Acylglycerol kinase inhibits macrophage anti-tumor activity via limiting mtDNA release and cGAS-STING-type I IFN response." Theranostics 15.4 (2025): 1304. https://www.thno.org/v15p1304.htm
Anti-AGK antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-DLG1 Monolconal Antibody (4F3) (CBMAB-0225-CN)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

-
Mouse Anti-ATG5 Recombinant Antibody (9H197) (CBMAB-A3945-YC)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-ALDOA Recombinant Antibody (A2) (CBMAB-A2316-YC)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-ADAM29 Recombinant Antibody (V2-179787) (CBMAB-A1149-YC)

-
Rabbit Anti-ALDOA Recombinant Antibody (D73H4) (CBMAB-A2314-YC)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Mouse Anti-CARTPT Recombinant Antibody (113612) (CBMAB-C2450-LY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-FN1 Monoclonal Antibody (D6) (CBMAB-1240CQ)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








