MFF Antibodies
Background
Mycophenolate mofetil (MMF) is a key immunosuppressive drug, primarily used to prevent organ transplant rejection and treat autoimmune diseases. Its active form, mycophenolic acid (MPA), selectively inhibits inosine monophosphate dehydrogenase (IMPDH), blocking purine synthesis crucial for lymphocyte growth. Unlike conventional immunosuppressants, MMF offers superior specificity with reduced bone marrow toxicity. It was first approved for kidney transplantation in the 1990s and has now become one of the core drugs in immunosuppressive regimens. MMF specifically inhibits the activation of T/B cells by precisely targeting the IMPDH enzyme. This unique mechanism not only improves the survival rate of transplanted organs, but also provides ideas for the development of new immunomodulatory drugs.
Structure of MMF
Mycophenolate mofetil (MMF), an immunosuppressive prodrug, is synthesized by esterifying mycophenolic acid (MPA)—a benzofuran-based compound—with 2-morpholinoethanol. This modification optimizes MPA's bioavailability while retaining its therapeutic activity.
The structure-function relationship of MMF:
- The 2-morpholinoethyl ester moiety, attached through an ester linkage, acts as a prodrug group that markedly enhances intestinal absorption, achieving over 90% oral bioavailability.
- The benzofuran core provides structural rigidity, and the methoxy substitution improves metabolic stability.
- The α,β-unsaturated carboxylic acid moiety acts as the key pharmacophore, directly binding to IMPDH's NAD⁺site to achieve selective inhibition.
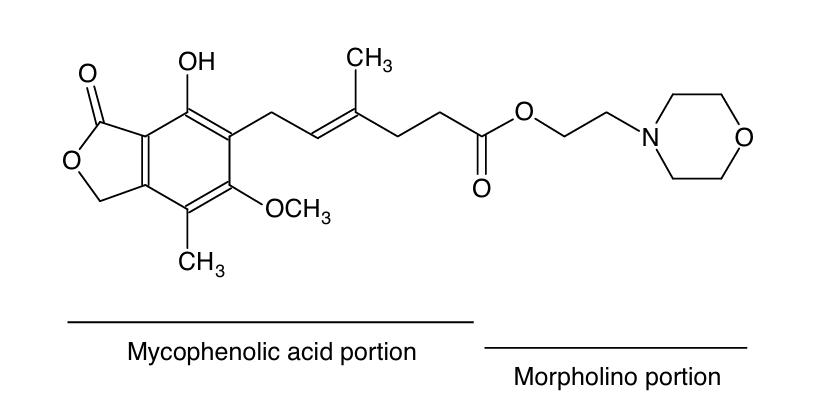 Fig. 1 Structural formula of mycophenolate mofetil.1
Fig. 1 Structural formula of mycophenolate mofetil.1
Key structural features of MMF:
- Prodrug-modified ester bond structure
- Rigid benzofuran skeleton
- α,β -unsaturated carboxylic acid pharmacophore
- Morpholine ring system
Functions of MMF
Mycophenolate mofetil (MMF) is a critical immunosuppressive agent that primarily targets lymphocyte proliferation. Its clinical applications include organ transplantation and autoimmune disease management, where it modulates immune responses and influences metabolic pathways in immune cells.
| Function | Description |
| Immunosuppression | Mycophenolic acid (MPA), the bioactive metabolite of MMF, selectively inhibits IMPDH, a key enzyme in guanine nucleotide synthesis. |
| Treatment of autoimmune diseases | It is used for the treatment of autoimmune diseases such as lupus nephritis and has better safety compared with cyclophosphamide. |
| Metabolic selectivity | It mainly affects lymphocytes that rely on the de novo synthesis pathway and has a relatively small impact on most somatic cells. |
| Pharmacokinetic characteristics | By converting to a prodrug, the compound demonstrates over 90% oral bioavailability. |
Compared with traditional immunosuppressants (such as azathioprine), MMF has a highly selective inhibitory effect on IMPDH. This specificity significantly reduces its bone marrow toxicity and makes it a core drug in modern immunosuppressive regimens.
Applications of MMF and MMF Antibody in Literature
1. Stumpf, Julian, et al. "MMF/MPA is the main mediator of a delayed humoral response with reduced antibody decline in kidney transplant recipients after SARS-CoV-2 mRNA vaccination." Frontiers in Medicine 9 (2022): 928542. https://doi.org/10.3389/fmed.2022.928542
This study identifies MMF/MPA as a critical determinant of impaired SARS-CoV-2 vaccine response in kidney transplant recipients, demonstrating significantly accelerated antibody decline within two months post-vaccination compared to non-MMF regimens.
2. Al Fatly, Z., et al. "Mycophenolate mofetil hampers antibody responses to a broad range of vacacinations in kidney transplant recipients: results from a randomized controlled study." Journal of Infection 88.3 (2024): 106133. https://doi.org/10.1016/j.jinf.2024.106133
Clinical studies demonstrate that mycophenolate mofetil (MMF) significantly impairs vaccine-induced antibody production in kidney transplant patients. MMF's immunosuppressive properties also correlate with diminished antibody responses to SARS-CoV-2 vaccines, highlighting its broad suppressive effect on post-transplant humoral immunity.
3. Kimball, Jess A., et al. "Reduced human IgG anti-ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil." Transplantation 60.12 (1995): 1379-1383. https://doi.org/10.1097/00007890-199560120-00001
Studies demonstrate that mycophenolate mofetil (MMF) significantly lowers anti-ATGAM antibody formation in kidney transplant recipients. MMF excels at suppressing potent antibody responses (0–20%), far outperforming azathioprine (56%). This represents the first clinical evidence of MMF's capacity to inhibit human B-cell mediated antibody responses in vivo, extending beyond its known T-cell suppressive effects.
4. Li, Shengde, et al. "Long-term efficacy of mycophenolate mofetil in myelin oligodendrocyte glycoprotein antibody-associated disorders: a prospective study." Neurology: Neuroimmunology & Neuroinflammation 7.3 (2020): e705.https://doi.org/10.1002/lt.24738
This study demonstrates the significant efficacy of mycophenolate mofetil (MMF) in preventing relapses in MOG antibody-associated disorders (MOGAD). MMF-treated patients showed a markedly lower relapse rate (7.4%) versus untreated cases (44.0%). The adjusted hazard ratio of 0.08 (95% CI 0.02–0.28) further confirms MMF's protective effect against disease recurrence.
5. Ersoy, Gulcin Sahin, et al. "Mycophenolate mofetil attenuates uterine ischaemia/reperfusion injury in a rat model." Reproductive BioMedicine Online 34.2 (2017): 115-123. https://doi.org/10.1016/j.rbmo.2016.11.007
This study demonstrates that mycophenolate mofetil (MMF) effectively mitigates uterine ischemia/reperfusion injury. MMF significantly reduced oxidative stress markers (8-OHdG, MDA, MPO) while enhancing SOD activity (*p*<0.01). Histological analysis confirmed attenuated tissue damage and apoptosis, indicating MMF's protective mechanisms via antioxidant and anti-inflammatory pathways.
Creative Biolabs: MMF Research Solutions
Creative Biolabs is dedicated to providing high-quality MMF antibodies and related research tools for pharmacological research and clinical applications.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MMF research products, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Bullingham, Roy ES, Andrew J. Nicholls, and Barbara R. Kamm. "Clinical pharmacokinetics of mycophenolate mofetil." Clinical pharmacokinetics 34.6 (1998): 429-455. https://doi.org/10.2165/00003088-199834060-00002
Anti-MFF antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-EIF4G1 Recombinant Antibody (2A9) (CBMAB-A2544-LY)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Rabbit Anti-CBL Recombinant Antibody (D4E10) (CBMAB-CP0149-LY)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-180650) (CBMAB-A2186-YC)

-
Rat Anti-ADGRE4 Recombinant Antibody (V2-160163) (CBMAB-F0011-CQ)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Rabbit Anti-CCL5 Recombinant Antibody (R0437) (CBMAB-R0437-CN)

-
Mouse Anti-AMOT Recombinant Antibody (CBYC-A564) (CBMAB-A2552-YC)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-DHFR Recombinant Antibody (D0821) (CBMAB-D0821-YC)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-CCNH Recombinant Antibody (CBFYC-1054) (CBMAB-C1111-FY)

-
Mouse Anti-ATP5F1A Recombinant Antibody (51) (CBMAB-A4043-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






