EED Antibodies
Background
EED (embryonic ectodermal development protein) is the core component of the PRC2 complex and mainly exists in the nucleus of eukaryotic cells. This protein regulates gene silencing by recognizing the H3K27me3 modification and maintains epigenetic memory. Tumor cells rely on EED to maintain an abnormal proliferation state. In the 1990s, scientists discovered EED in fruit flies. The β -propeller formed by its WD40 domain can specifically recognize H3K27me3, and this discovery lays the foundation for epigenetic research. The three-dimensional structure of EED reveals its unique aromatic amino acid binding pockets. The ingenious structure and regulatory mechanism of EED make it an ideal model for studying protein-protein interactions and epigenetics. At present, inhibitors targeting EED have become a new direction in the research and development of anti-tumor drugs.
Structure of EED
EED is a 45-50kDa nuclear protein featuring a WD40 repeat-derived β-propeller architecture that facilitates H3K27me3 recognition and PRC2 complex assembly.
The structure-function relationship of EED:
1.WD40 repeating domain: Composed of 7 WD40 repeating sequences, it forms a stable β-propeller structure, creating the protein-protein interaction interface required for the assembly of the PRC2 complex.
2.Aromatic binding pocket: This specialized domain contains conformed aromatic amino acid residues, which can specifically recognize and bind methylated histone markers (especially H3K27me3).
3.C-terminal extension region: By regulating the stability of the PRC2 complex and acting in synergy with EZH2, it regulates the catalytic efficiency of methyltransferase.
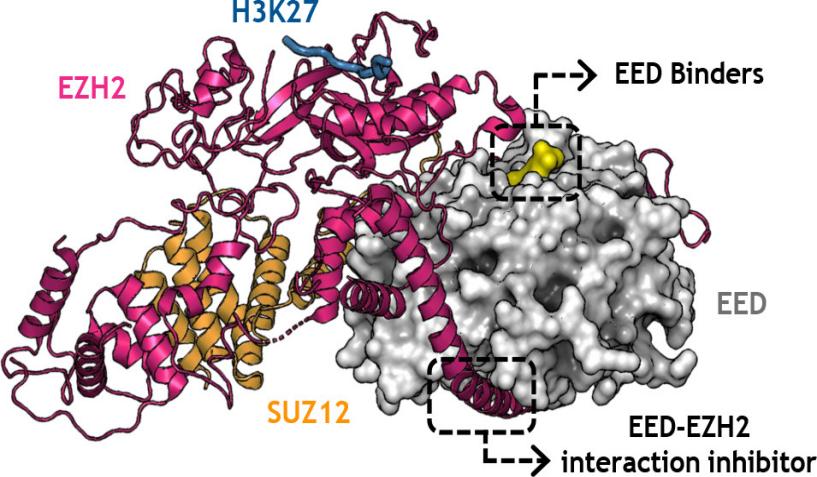 Fig. 1 EZH2, EED, and H3K27 Interaction Inhibitors in Epigenetic Regulation.1
Fig. 1 EZH2, EED, and H3K27 Interaction Inhibitors in Epigenetic Regulation.1
Functions of EED
As the structural organizer of PRC2, EED mediates three fundamental epigenetic processes:
| Function | Description |
| Transcriptional Silencing | Directs PRC2 to chromatin through H3K27me3 recognition, establishing heritable repressive chromatin domains. |
| Chromatin Compaction | Facilitates higher-order chromatin organization by nucleating heterochromatin formation and maintaining topological domains. |
| Lineage Commitment | Orchestrates stem cell fate transitions by dynamically controlling pluripotency and differentiation gene networks. |
Signal pathway regulation:
The latest research indicates that EED participates in the regulation of multiple signaling pathways through non-classical approaches.
- The Wnt/β-catenin pathway
- The NF-κB inflammatory pathway
- The PI3K/AKT/mTOR growth regulatory pathway
The key role of EED in the occurrence and development of diseases:
- The abnormal expression of EED is closely related to many major diseases. In hematological malignancies, EED mutations are important drivers of myelodysplastic syndrome and T-cell lymphoma
- In terms of fibrotic diseases, EED participates in the fibrotic process of organs such as the lungs, liver and kidneys by regulating the activation of myofibroblasts and extracellular matrix deposition. The latest research has found that inhibiting the activity of EED can significantly improve fibrotic symptoms. In addition, the dysregulation of EED expression in adipose tissue can also lead to insulin resistance and obesity. Knocking out EED can improve metabolic disorders
- EED dysfunction contributes to neuropathology and autoimmunity through epigenetic control of neuroprotective and immunomodulatory gene networks. These findings indicate that EED is a target with broad therapeutic potential, but it also faces the challenge of specific regulation. Unlike myoglobin antibodies that mainly target muscle diseases, targeting EED requires more precise intervention in the complex epigenetic regulatory network
The oxygen dissociation curve of myoglobin is hyperbolic in contrast to hemoglobin's sigmoidal curve, indicating its higher oxygen affinity and role as a short-term oxygen reservoir.
Applications of EED and EED Antibody in Literature
1. Qi, Wei, et al. "An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED." Nature chemical biology 13.4 (2017): 381-388. https://doi.org/10.1038/nchembio.2304
EED226 is a first-in-class allosteric PRC2 inhibitor targeting EED's H3K27me3-binding pocket. It disrupts PRC2's methyltransferase activity, inhibiting H3K27me3 deposition. Unlike SAM-competitive inhibitors, EED226 maintains efficacy against therapy-resistant EZH2 mutants, offering new therapeutic potential for PRC2-driven malignancies.
2. Cook, Nicholas, et al. "Embryonic ectoderm development (EED) as a novel target for cancer treatment." Current topics in medicinal chemistry 21.31 (2021): 2771-2777. https:// doi.org/10.2174/1568026621666210920154942
EED serves as a critical structural scaffold within PRC2, where its H3K27me3-binding activity allosterically enhances EZH2's catalytic function. Pharmacological targeting of EED's regulatory domain disrupts PRC2 integrity and epigenetic silencing capacity.
3. Liu, Pei‐Pei, et al. "An EED/PRC2‐H19 Loop Regulates Cerebellar Development." Advanced Science 12.1 (2025): 2403591. https:// doi.org/10.1002/advs.202403591
The EED-H19 axis critically regulates cerebellar morphogenesis through an epigenetic feedback mechanism. EED deficiency impairs granule cell progenitor proliferation via H3K27me3-dependent suppression of the lncRNA H19, whose subsequent overexpression reciprocally inhibits EED, establishing a regulatory circuit essential for proper cerebellar development.
4. Ying, Xiuru, et al. "Significant association of EED promoter hypomethylation with colorectal cancer." Oncology Letters 18.2 (2019): 1564-1570. https:// doi.org/10.3892/ol.2019.10432
Genome-wide analysis revealed significant EED promoter hypomethylation in CRC tissues (5.03% vs 8.65% in adjacent and 40.12% in normal tissues, p<0.01). Functional assays demonstrated the EED regulatory element's 3.15-fold transcriptional enhancement (p=0.014), implicating EED epigenetic dysregulation in CRC pathogenesis.
5. Zhao, Yuan, et al. "Recent strategies targeting Embryonic Ectoderm Development (EED) for cancer therapy: Allosteric inhibitors, PPI inhibitors, and PROTACs." European Journal of Medicinal Chemistry 231 (2022): 114144. https:// doi.org/10.1016/j.ejmech.2022.114144
The core protein EED of the PRC2 complex regulates gene silencing by recognizing the H3K27me3 marker, and its abnormal activation is closely related to various cancers. Novel strategies targeting EED, such as allosteric inhibitors, protein-protein interaction blockers and PROTAC degraders, provide a breakthrough direction for overcoming the drug resistance and indication limitations of existing EZH2 inhibitors.
Creative Biolabs: EED Antibodies for Research
Creative Biolabs is dedicated to providing high-quality EED (Embryonic Ectodermal Development Protein) antibodies, serving the fundamental research in epigenetics and the development of tumor treatments. Our antibody products have been strictly verified and can be precisely applied to: ELISA, Flow cytometry (FC), Western Blot (WB), or Immunohistochemistry (IHC).
- Custom EED antibody Development: Personalized solutions based on your experimental needs.
- Bulk Production: To meet the bulk requirements of industrial partners.
- Technical Support: Provide scheme optimization and problem diagnosis services.
- Aliquoting Services: Small subpacking ensures long-term stability of antibodies.
For more details on our EED antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Tomassi, Stefano, et al. "Polycomb repressive complex 2 modulation through the development of EZH2–EED interaction inhibitors and EED binders." Journal of Medicinal Chemistry 64.16 (2021): 11774-11797. https://doi.org/10.1021/acs.jmedchem.1c00226
Anti-EED antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ADAM29 Recombinant Antibody (V2-179787) (CBMAB-A1149-YC)

-
Mouse Anti-BHMT Recombinant Antibody (CBYY-0547) (CBMAB-0550-YY)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-CARD11 Recombinant Antibody (CBFYC-0811) (CBMAB-C0866-FY)

-
Mouse Anti-DLG1 Monolconal Antibody (4F3) (CBMAB-0225-CN)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-AGK Recombinant Antibody (V2-258056) (CBMAB-M0989-FY)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






