IgM
Background
As the largest immunoglobulin isotype by molecular weight, IgM demonstrates unique structural and functional attributes critical to immune defense mechanisms. Its characteristic pentameric configuration, stabilized through J-chain-mediated polymerization, enables high-avidity antigen interactions and potent complement cascade initiation. As the first immunoglobulin class generated during humoral immune responses, IgM exhibits preferential binding to conserved pathogen-associated molecular patterns through multivalent epitope recognition, a feature leveraged in diagnostic evaluations of acute-phase infections. Furthermore, IgM's Fc-mediated opsonization capacity enhances phagocytic clearance while simultaneously modulating B-cell receptor signaling through membrane-bound interactions. Pathological analyses reveal distinct correlations between aberrant IgM expression profiles and specific B-cell neoplastic disorders, underscoring its dual roles in immune regulation and disease pathogenesis.
Structure of IgM
As the predominant multimeric antibody in primary immune responses, IgM exhibits a pentavalent architecture stabilized through J-chain polymerization, conferring exceptional antigen-binding avidity. This macromolecular organization integrates four hierarchical levels: (1) A μ-heavy chain backbone containing variable (VH) and constant (CH1-4) domains linked by disulfide bridges; (2) Ten antigen-binding fragments formed through VH/VL domain pairing; (3) A central J-chain scaffold coordinating pentameric assembly via CH3 domain interactions; (4) Complement-activating effector modules within the Fc5μ region. The quaternary configuration enables simultaneous pathogen neutralization through multivalent epitope engagement while maintaining structural flexibility for immune complex formation, with cryo-EM studies demonstrating <4Å resolution of its hinge-mediated domain reorientation during opsonization processes.
- Primary Structure:The μ heavy chain contains four constant domains (CH1-CH4) and one variable domain (VH), with the CH4 domain critical for complement activation. Each light chain pairs with a heavy chain via disulfide bonds, forming antigen-binding fragments (Fab). A cysteine-rich joining (J) chain links two μ heavy chains at the CH3 domains, stabilizing the pentameric assembly.
- Secondary Structure:Immunoglobulin-fold domains dominate the structural organization, formed by β-sheet sandwich motifs stabilized by intrachain disulfide bonds. The Fab regions exhibit β-barrel configurations in variable domains, enabling conformational flexibility for antigen binding. In the Fc region, parallel β-sheets and α-helices create rigid frameworks for effector molecule interactions.
- Functional Domains:The antigen-binding fragment (Fab) is composed of the heavy chain and light chain variable regions, and its complementary determining regions (CDRs) realize specific antigen epitope recognition through highly variable loop structures; the effector fragment (Fc) has a conserved glycosylation site (Asn402) at the CH2-CH3 domain interface, which mediates the binding and activation of complement C1q molecules through spatial conformational exposure; the connecting chain (J chain) is a 15kDa polypeptide hub, which stabilizes the spatial assembly of immunoglobulins with the help of pentamer cross-linking mediated by cysteine residues, and promotes the transcellular transport of polymers across the mucosal epithelium through polar residue clusters. These structural elements work together at multiple levels, enabling IgM to simultaneously possess efficient antigen capture ability, complement cascade initiation efficacy, and mucosal immune barrier penetration characteristics.
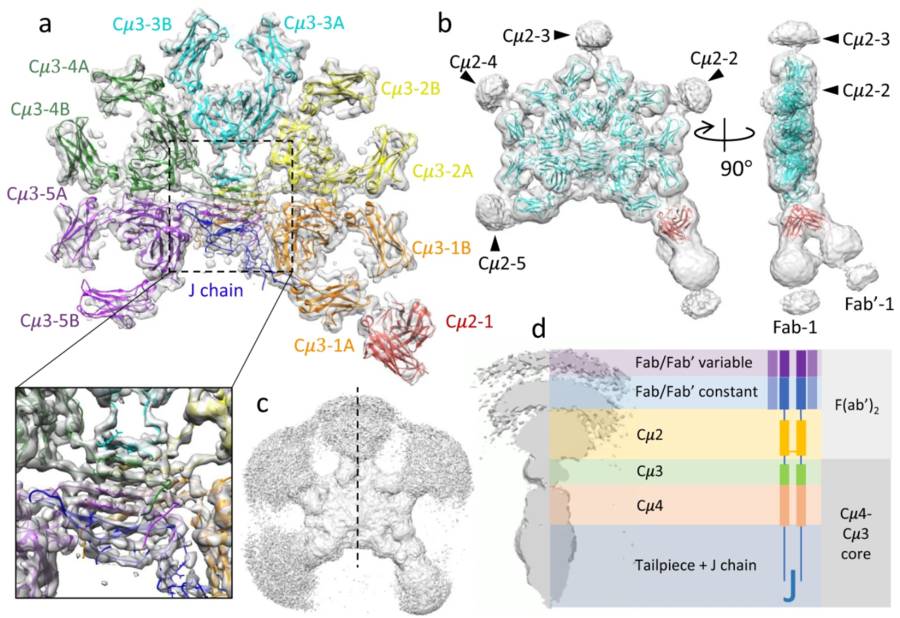 Fig. 1 The cryo-EM structure of FL-IgM.1
Fig. 1 The cryo-EM structure of FL-IgM.1
Functions of IgM
As the core effector molecule of humoral immunity, IgM realizes a multi-level immune protection mechanism through its unique pentamer configuration. This immunoglobulin takes the lead in neutralizing pathogens in the primary immune response, and its ten antigen-binding sites significantly improve the efficiency of antigen capture through spatial synergy. The complement activation function depends on the conservative glycosylation modification of the Fc region, which can quickly trigger the cascade reaction of the classical pathway to form a membrane attack complex to directly lyse pathogens. In the field of pathological diagnosis, dynamic monitoring of serum IgM levels provides an early warning indicator for acute infection, while abnormal expression of monoclonal IgM is closely related to B-cell malignancies such as Waldenstrom's macroglobulinemia. In recent years, IgM-based immune complex engineering has been applied to the development of new vaccine adjuvants, and its powerful opsonization and phagocytosis enhancement effect has opened up a new path for the treatment of intracellular bacterial infections. This multifunctional characteristic of transformation from innate immune defense to clinical diagnosis and treatment has established the hub position of IgM in the immune system.
Applications of IgM and IgM Antibody in Literature
1. Korell, Felix et al. "First third-generation CAR T cell application targeting CD19 for the treatment of systemic IgM AL amyloidosis with underlying marginal zone lymphoma." Biomarker research 11.1 (2025):91. https://doi.org/10.1186/s40364-023-00532-2
This study identifies immunoglobulin M (IgM) paraprotein as a critical mediator in B-cell lymphoma-associated light chain amyloidosis pathogenesis, and demonstrates the clinical efficacy of third-generation CD19-directed CAR T-cell therapy in achieving sustained hematological remission for refractory AL amyloidosis patients with active IgM-secreting malignancies.
2. Webster, Sarah E et al. "Secreted IgM deficiency alters the retinal landscape enhancing neurodegeneration associated with aging." Immunity & ageing : I & A 22.1 (2025):9. https://doi.org/10.1186/s12979-025-00502-2
This study demonstrates that secreted immunoglobulin M (IgM) critically sustains retinal neuro-glial homeostasis during aging, and reveals its protective capacity in mitigating blood-retinal barrier dysfunction and proinflammatory cytokine cascades, positioning B-cell-derived natural antibodies as potential therapeutic targets for age-related macular degeneration and degenerative retinopathies.
3. Romera, Clara et al. "Mouse brain contains age-dependent extraparenchymal granular structures and astrocytes, both reactive to natural IgM antibodies, linked to the fissura magna." Immunity & ageing : I & A 21.1 (2024):56. https://doi.org/10.1186/s12979-024-00460-1
This study identifies age-associated IgM-reactive carbohydrate neoepitopes in murine brain structures, and reveals their critical involvement in cerebrospinal immunosurveillance through the characterization of two novel IgM+ complexes—fibroblast-associated EP granules in extraparenchymal fissures and glial-bound astrocytic aggregates—that exhibit dynamic accumulation patterns correlating with cerebral clearance mechanisms.
4. Wang, Fengmei et al. "Clinical relevance of glomerular IgM deposition in patients with lupus nephritis." BMC immunology 22.1 (2021):75. https://doi.org/10.1186/s12865-021-00467-z
This study demonstrates that glomerular IgM deposits critically potentiate complement-mediated renal injury in lupus nephritis (LN) through pathological synergy with complement factor H (CFH) deficiency, and establishes their diagnostic value as independent predictors of C3 hyperactivation and progressive renal dysfunction in LN patients.
5. Nguyen, Lee S et al. "Sensitivity of point-of-care IgM and IgG test in critically ill patients with SARS-Cov-2." Critical care (London, England) 24.1 (2020):573. https://doi.org/10.1186/s13054-020-03290-x
This study demonstrates that IgM detection via point-of-care serological testing (POCST) exhibits compromised sensitivity in critically ill SARS-CoV-2 ICU patients due to immunosuppression-related humoral response attenuation, while confirming its technical feasibility as a rapid triage adjunct when combined with standardized RT-PCR confirmation protocols.
Creative Biolabs: IgM Antibodies for Research
As a professional supplier in the field of immunoglobulins, we focus on providing full-spectrum IgM antibody solutions, especially monoclonal antibody product lines, meeting industrial-grade stability and batch consistency requirements. Advantageous service modules include:
- Intelligent customized development: Optimizing antibody affinity and specificity based on antigen epitope characteristics
- Flexible production: Supporting seamless connection from milligram-level R&D trials to kilogram-level industrial supply
- Stability guarantee: Freeze-dried preparations and liquid nitrogen storage technology ensure long-term maintenance of antibody activity
Global logistics support: Temperature-controlled packaging system enables safe transportation in all climate zonesFor more details on our IgM antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Chen, Qu et al. "Cryomicroscopy reveals the structural basis for a flexible hinge motion in the immunoglobulin M pentamer." Nature communications 13.1 (2022):6314. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1038/s41467-022-34090-2
Anti-IgM antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

-
Mouse Anti-ATP5F1A Recombinant Antibody (51) (CBMAB-A4043-YC)

-
Mouse Anti-CGAS Recombinant Antibody (CBFYM-0995) (CBMAB-M1146-FY)

-
Mouse Anti-CFL1 Recombinant Antibody (CBFYC-1771) (CBMAB-C1833-FY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Mouse Anti-ASB9 Recombinant Antibody (1D8) (CBMAB-A0529-LY)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Mouse Anti-CALR Recombinant Antibody (CBFYC-0763) (CBMAB-C0818-FY)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-Acetyl SMC3 (K105/K106) Recombinant Antibody (V2-634053) (CBMAB-AP052LY)

-
Mouse Anti-CA9 Recombinant Antibody (CBXC-2079) (CBMAB-C0131-CQ)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






