OGT Antibodies
Background
O-linked N-acetylglucosamine transferase (OGT) exists as one of the crucial enzymes in O-linked-N-acetylglucosaminylation (O-GlcNAcylation), a significant post-translational modification (PTM) related to the development of cancer. The process includes the enzyme-mediated link between O-linked β-N-acetylglucosamine (O-GlcNAc) and serine/threonine residues in proteins. First discovered in 1984 by Gerald W. Hart, OGT was proven to be involved in various physiological process, including nervous system development, the regulation of mammalian cell and hematopoietic stem cell homeostasis. Its elevated expression in multiple tumors suggests a potential pro-cancer role.
Structure of OGT
OGT is recognized as a member of the CAZy GT-B glycosyltransferase family encoded by a single mammalian gene on the X chromosome. The human genome encodes three distinct OGT isoforms, each characterized by unique variations in their N-terminal TPR domain and differential intracellular distribution patterns.
| Species | Human | Human | Human |
| Subtypes | Nucleocytoplasmic OGT (ncOGT) | Mitochondrial OGT (mOGT) | Short OGT (sOGT) |
| Molecular Weight (kDa) | 116 | 103 | 78 |
| Primary Structural Differences | 13.5 TPRs at the N-terminus located in the nucleus and cytoplasm | 9.5 TPRs at the N-terminus located in mitochondrion | 2.5 TPRs at the N-terminus located in cytoplasm |
The enzyme OGT and the enzyme O-GlcNAcase (OGA) are one pair of enzymes that mediate the addition and removal of O-GlcNAc on proteins. The enzyme OGT comprises an N-terminall domain containing varying TPR mediated protein-protein interactions and a C-terminal catalytic domain responsible for its glycosyltransferase activity. Two critical structural determinants of OGT: (i) an asparagine ladder that stabilizes substrate binding through peptide backbone interactions; (ii) a cluster of luminal aspartate residues (Asp386, Asp420, Asp454) that precisely coordinate glycosylation site selection.
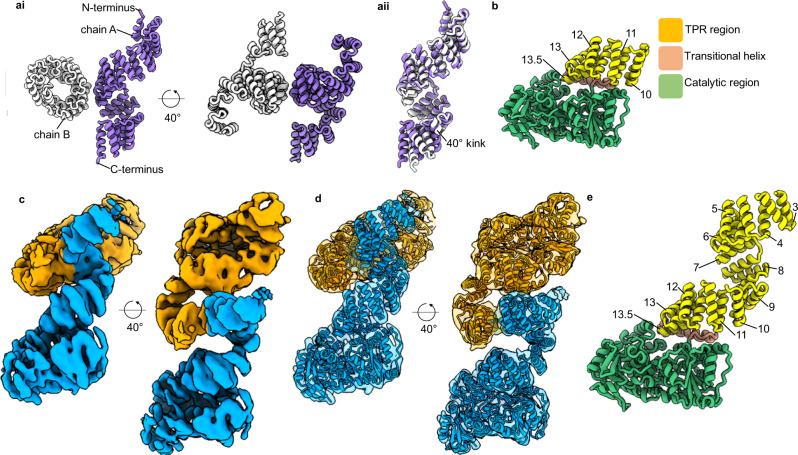 Fig. 1 The structure of unliganded OGT.1
Fig. 1 The structure of unliganded OGT.1
Functions of OGT
OGT primarily participates in cell cycle regulation, mitochondrial energy metabolism and PTM. However, its involvement in cancer development through 6 ways has also been implicated in various years.
| Roles of OGT in cancer progression | Description |
| Cancer Proliferation | OGT increases the expression of proliferation-related factors facilitating the abnormal proliferation in cancers including hepatocellular carcinoma, gastric cancer and breast cancer. |
| Cancer Invasion and Metabolism | Apart from the acceleration of related factor expression, OGT also plays a role in epithelial-mesenchymal transition (EMT) and matrix metalloproteinase regulation, thereby affecting tumor invasion and metastasis. |
| Cell Death | Evidences suggested that OGT get involved in apoptosis, ferroptosis and autophagy. |
| Cancer Stem-like Cells Properties | Restoring the tumor stem cell phenotype through OGT is expected to provide an effective strategy for tumor treatment. |
| Immune Escape | Research indicates OGT-mediated post-translational modifications protect PD-L1 from proteasomal destruction, thereby enabling immune evasion in cancer. |
| Drug resistance | Knockdown of OGT reduce the cancer cell sensitivity to drug treatment in partial cancers (colorectal cancer et al.), while OGT knockdown enhance the drug sensitivity in various cancers (bladder cancer et al.). |
Applications of OGTand OGT Antibody in Literature
1. Tang, Jianing, et al. "Targeting USP8 inhibits O‐GlcNAcylation of SLC7A11 to promote ferroptosis of hepatocellular carcinoma via stabilization of OGT." Advanced Science 10.33 (2023): 2302953. https://doi.org/10.1002/advs.202302953
The article highlights OGT as an accelerating factor in the progression of hepatocellular carcinoma (HCC) and introduces an anti-tumor treatment,deubiquitylating enzymes (DUB), which induces ferroptosis and decreases tumor cell viability via decreasing the stability of OGT, potentially serving as a promising anti-tumor strategy for HCC.
2. Zhou, Qianqian, et al. "KIF1A promotes neuroendocrine differentiation in prostate cancer by regulating the OGT-mediated O-GlcNAcylation." Cell Death & Disease 15.11 (2024): 796. https://doi.org/10.1038/s41419-024-07142-2
This article demonstrates that OGT plays a crucial role in the transition from prostate adenocarcinoma after failed endocrine treatment to neuroendocrine prostate cancer (NEPC), inducing higher tumor lethality. An OGT inhibitor, OSMI-1, can significantly suppress KIF1A-mediated PCa cell proliferation in vitro and tumor growth in vivo.
3. Jin, Haoyi, et al. "Mannose inhibits NSCLC growth and inflammatory microenvironment by regulating gut microbiota and targeting OGT/hnRNP R/JUN/IL-8 Axis." International Journal of Biological Sciences 21.4 (2025): 1566. https://doi.org/10.7150/ijbs.107256
This article utilizes multi-omics and cellular experiments to investigate the intrinsic mechanism of the anti-tumor effect of mannose on NSCLC. Evidences suggest that OGT directly mediates the anti-tumor and anti-inflammatory function of mannose.
4. Loison, Ingrid, et al. "O-GlcNAcylation inhibition redirects the response of colon cancer cells to chemotherapy from senescence to apoptosis." Cell Death & Disease 15.10 (2024): 762. https://doi.org/10.1038/s41419-024-07131-5
This article develops a complex therapy combining low doses of conventional chemotherapeutic drugs with OGT inhibitors like OSMI-4, achieving preserved efficacy together with reduced side effects specialized in colorectal cancer with higer O-GlcNAc levels.
5. Yang, Yang, et al. "O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N6-methyladenosine-dependent manner." Signal Transduction and Targeted Therapy 8.1 (2023): 63. https://doi.org/10.1038/s41392-023-01316-8
This article describes the potential therapeutic targets of hepatocellular carcinoma (HCC), where OCT-mediated YTHDF2 O-GLcNAcylation can be suppressed by OSMI-6 (an OCT inhibitor) to inhibit the HBV-related HCC tumor growth, demonstrating its diagnostic potential in HBV-related HCC.
Company A: OGT Antibodies for Research
Company A specializes in the production of high-quality OGT antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom OGT Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our OGT antibodies, custom preparations, or technical support, contact us at email.
Reference
- Meek, Richard W., et al. "Cryo-EM structure provides insights into the dimer arrangement of the O-linked β-N-acetylglucosamine transferase OGT." Nature Communications 12.1 (2021): 6508. https://doi.org/10.1038/s41467-021-26796-6
Anti-OGT antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-ATP1B3 Recombinant Antibody (1E9) (CBMAB-A4021-YC)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

-
Mouse Anti-ENO2 Recombinant Antibody (H14) (CBMAB-E1341-FY)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Mouse Anti-ARIH1 Recombinant Antibody (C-7) (CBMAB-A3563-YC)

-
Mouse Anti-CA9 Recombinant Antibody (CBXC-2079) (CBMAB-C0131-CQ)

-
Mouse Anti-DDC Recombinant Antibody (8E8) (CBMAB-0992-YC)

-
Mouse Anti-CEMIP Recombinant Antibody (3C12) (CBMAB-K0296-LY)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Rabbit Anti-ENO2 Recombinant Antibody (BA0013) (CBMAB-0272CQ)

-
Mouse Anti-ENO1 Recombinant Antibody (CBYC-A950) (CBMAB-A4388-YC)

-
Mouse Anti-DHFR Recombinant Antibody (D0821) (CBMAB-D0821-YC)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Rat Anti-CD300A Recombinant Antibody (172224) (CBMAB-C0423-LY)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








