SYK Antibodies
Background
SYK is a cytoplasmic signal transduction enzyme mainly located in hematopoietic system cells, which mediates specific phosphorylation cascade reactions through its tandem SH2 domains. The kinase triggers the transmembrane signal transduction mechanism by recognizing the phosphorylation sites of the immunoreceptor tyrosine activation motifs (ITAMs), and plays a core regulatory role in B cell receptor signal activation and FcεRI-mediated allergic response pathways. Its unique autoinhibitory dual-domain structure (through the steric hindrance effect of the N-terminal SH2 domain and the kinase domain) has been analyzed by X-ray crystallography, revealing the molecular switch mechanism of kinase activity induced by substrate binding conformational changes.
Structure of SYK
SYK (spleen tyrosine kinase) displays a hierarchical structural architecture comprising three functionally integrated modules. Positioned at the amino terminus, tandem SH2 domains (residues 1-261) operate as phosphorylation-responsive elements, while the carboxyl-terminal catalytic domain (residues 344-635) functions as the enzymatic core. These components are interconnected via an 83-residue linker peptide that facilitates dynamic interdomain communication. Advanced structural characterization reveals that conserved αA-αC helices within the SH2 domains utilize arginine-phosphate electrostatic interactions for selective recognition of ITAM-phosphorylated tyrosines. The kinase domain exhibits a distinctive bilobal architecture: the N-lobe (residues 344-484) assembles an ATP-binding groove through antiparallel β-sheet (β1-β5) and αC-helix arrangements, while the C-lobe (residues 485-635) employs αD-αH helical assemblies to stabilize catalytic site geometry.
Activation proceeds through sequential conformational transitions initiated by dual SH2 engagement with phosphorylated ITAM motifs. Cooperative binding via FLVR sequences (residues 110-113/231-234) induces a 25° interdomain rotation that disrupts intramolecular inhibition. Critical phosphorylation events at linker residues Y342/Y346 alleviate steric constraints between SH2 and kinase domains, enabling the activation loop (residues 525-555) to transition from a catalytically inert, closed state to an active conformation. This structural rearrangement is stabilized by tripartite hydrogen bonding between phosphorylated Y525/Y526 and conserved aspartate residues (D512/D544), effectively reconfiguring the catalytic cleft. The elucidated allostery-driven activation cascade has enabled rational development of therapeutic agents targeting the dynamic interface between the activation loop and αC-helix.
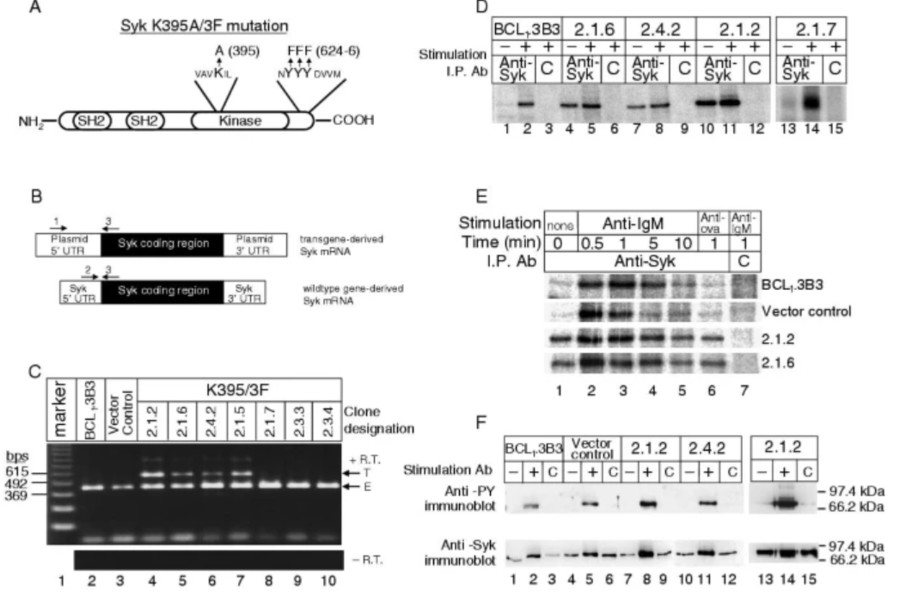 Fig. 1 Expression of the (K395A/3F)Syk molecule is associated with constitutive Syk kinase activity.1
Fig. 1 Expression of the (K395A/3F)Syk molecule is associated with constitutive Syk kinase activity.1
Functions of SYK
SYK kinase emerges as a pivotal regulatory nexus in immunological processes, exhibiting bifunctional properties that span physiological homeostasis and disease pathogenesis. Within canonical immune signaling, this enzyme operates through two complementary mechanisms: (1) as a principal mediator of B-cell receptor (BCR) and Fc receptor signaling cascades, where ligand engagement initiates intracellular calcium transients and NF-κB nuclear translocation, driving lymphocytic differentiation and antibody isotype switching; (2) as a modulator of innate immune responses, coordinating FcγR-mediated phagosomal maturation in macrophages while calibrating IL-1β production through phosphorylation-dependent control of NLRP3 inflammasome activation. This dual regulatory capacity enables SYK to orchestrate crosstalk between adaptive and innate immunity through spatiotemporal control of immune synapse formation and cytokine secretion dynamics.
Applications of SYK and SYK Antibody in Literature
1. Fan, Zhenzhen et al. "Metformin attenuates central sensitization by regulating neuroinflammation through the TREM2-SYK signaling pathway in a mouse model of chronic migraine." Journal of neuroinflammation 21.1 (2024):318. https://doi.org/10.1186/s12974-024-03313-2
The research identifies SYK as a critical mediator of TREM2-dependent neuroinflammation in chronic migraine pathology, and reveals metformin's dual-action mechanism suppressing SYK signaling to disrupt microglial activation and central pain sensitization.
2. Liao, Cheng et al. "Selective inhibition of spleen tyrosine kinase (SYK) with a novel orally bioavailable small molecule inhibitor, RO9021, impinges on various innate and adaptive immune responses: implications for SYK inhibitors in autoimmune disease therapy." Arthritis research & therapy 15.5 (2013):R146. https://doi.org/10.1186/ar4329
This study demonstrates that the orally bioavailable SYK inhibitor RO9021 (6-[(1R,2S)-2-Amino-cyclohexylamino]-4-(5,6-dimethyl-pyridin-2-ylamino)-pyridazine-3-carboxylic acid amide) exerts dual immunomodulatory effects by suppressing both BCR/Fc receptor signaling and TLR9-mediated type I interferon production, effectively attenuating arthritis progression in murine collagen-induced models through coordinated inhibition of B-cell differentiation and osteoclastogenesis.
3. Guillet, Éléonore et al. "Human dendritic cell maturation induced by amorphous silica nanoparticles is Syk-dependent and triggered by lipid raft aggregation." Particle and fibre toxicology 20.1 (2023):12. https://doi.org/10.1186/s12989-023-00527-9
This study identifies Syk kinase as a pivotal mediator in SAS nanoparticle-induced dendritic cell maturation, and reveals their mechanism through lipid raft aggregation triggering Src-dependent Syk activation to amplify pro-inflammatory T-cell responses.
4. Chen, Xuejiao et al. "SYK promotes the formation of neutrophil extracellular traps by inducing PKM2 nuclear translocation and promoting STAT3 phosphorylation to exacerbate hepatic ischemia-reperfusion injury and tumor recurrence." Molecular medicine (Cambridge, Mass.) 30.1 (2024):146. https://doi.org/10.1186/s10020-024-00907-7
This study demonstrates that SYK kinase exacerbates hepatic ischemia-reperfusion injury and tumor recurrence through dual neutrophil-macrophage crosstalk, and reveals SYK inhibition as a therapeutic strategy to disrupt PKM2/STAT3-driven NETosis and NLRP3 inflammasome hyperactivation in myeloid cells.
5. Makhoul, Stephanie et al. "cAMP- and cGMP-elevating agents inhibit GPIbα-mediated aggregation but not GPIbα-stimulated Syk activation in human platelets." Cell communication and signaling: CCS 17.1 (2019):122. https://doi.org/10.1186/s12964-019-0428-1
The research elucidates Syk's dual phosphorylation mechanism in GPIbα-initiated thrombus formation, and identifies counterintuitive PKA/PKG-mediated amplification of Syk signaling concurrent with inhibition of calcium mobilization and αIIbβ3 activation, proposing targeted Syk modulation as a precision strategy for antithrombotic intervention.
Creative Biolabs: SYK Antibodies for Research
Creative Biolabs focuses on providing high-affinity SYK antibodies and supporting solutions. The product line has been strictly verified by quality control, and has the characteristics of excellent batch-to-batch stability and excellent solubility performance, and is suitable for various in vitro research systems.
For more details on our SYK antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Hsueh, Robert C et al. "Activation of the Syk tyrosine kinase is insufficient for downstream signal transduction in B lymphocytes." BMC immunology 3 (2002):16. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1186/1471-2172-3-16
Anti-SYK antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-BCL2L1 Recombinant Antibody (H5) (CBMAB-1025CQ)

-
Mouse Anti-ATP1B1 Recombinant Antibody (E4) (CBMAB-0463-LY)

-
Mouse Anti-CCL18 Recombinant Antibody (64507) (CBMAB-C7910-LY)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Rabbit Anti-ABL1 (Phosphorylated Y185) Recombinant Antibody (V2-443434) (PTM-CBMAB-0001YC)

-
Mouse Anti-AP4E1 Recombinant Antibody (32) (CBMAB-A2996-YC)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503417) (CBMAB-V208-1369-FY)

-
Mouse Anti-FN1 Monoclonal Antibody (71) (CBMAB-1241CQ)

-
Mouse Anti-CA9 Recombinant Antibody (CBXC-2079) (CBMAB-C0131-CQ)

-
Rat Anti-EPO Recombinant Antibody (16) (CBMAB-E1578-FY)

-
Mouse Anti-COL12A1 Recombinant Antibody (CBYY-C3117) (CBMAB-C4560-YY)

-
Rabbit Anti-CBL Recombinant Antibody (D4E10) (CBMAB-CP0149-LY)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Mouse Anti-FLT1 Recombinant Antibody (11) (CBMAB-V0154-LY)

-
Rat Anti-AChR Recombinant Antibody (V2-12500) (CBMAB-0990-CN)

-
Mouse Anti-FAS2 Monoclonal Antibody (1D4) (CBMAB-0071-CN)

-
Mouse Anti-HTLV-1 gp46 Recombinant Antibody (CBMW-H1006) (CBMAB-V208-1154-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






