Evolving Antibody Therapies: Targeting Type 1 Diabetes
Type 1 diabetes mellitus (T1D) is an autoimmune disease resulting from the destruction of β-cells in the pancreatic islets due to the loss of tolerance to β-cell antigens (such as proinsulin, insulin, and glutamic acid decarboxylase 65 (GAD65)) by the peripheral vasculature. Both genetic and uncertain environmental factors influence T1D susceptibility, but CD4+ and CD8+ T cells are generally considered the primary driving forces behind β-cell destruction in T1D patients. When functional β-cell mass is reduced by about 80%, insulin production becomes insufficient to regulate glucose levels in the body. Currently, there is no established treatment for T1D, but the blood glucose level can be controlled and monitored by daily exogenous insulin therapy. Inadequate control of daily blood sugar levels can lead to serious complications.
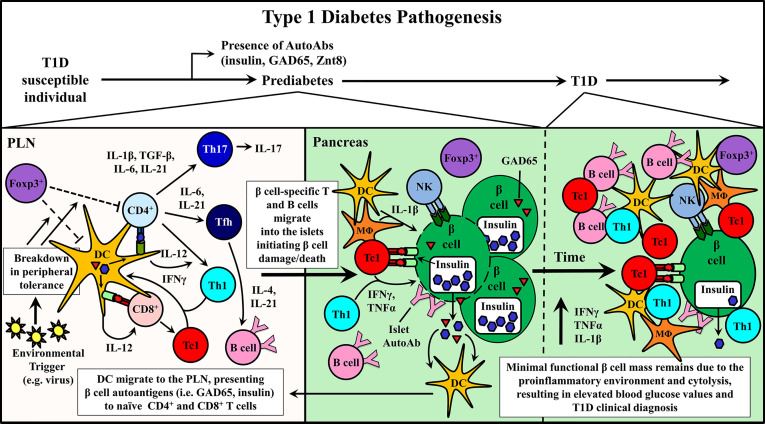 Fig. 1 Pathogenesis of Type 1 Diabetes Mellitus (T1D).1,3
Fig. 1 Pathogenesis of Type 1 Diabetes Mellitus (T1D).1,3
Monoclonal antibody therapy is an effective approach that has been clinically tested for the prevention and/or treatment of T1D and other autoimmune diseases. Clinical application of monoclonal antibodies and related molecules provides safe and selective therapeutic targeting of biologically relevant proteins, used to treat diverse diseases from cancer to autoimmunity. In T1D, mAb therapy must inhibit ongoing β-cell destruction while rebuilding long-term self-tolerance. The timing of T1D immunotherapy is considered a key factor affecting the clinical efficacy. Intervening with monoclonal antibodies in the early stages of β-cell autoimmunity, when the frequency of pathogenic immune effectors infiltrating the islets is relatively low and functional β-cell mass is high, is expected to be the most effective time to regulate autoimmune response. Alternatively, if therapy is initiated later, combining monoclonal antibody therapy with strategies to enhance the expansion and functionality of residual β-cell clusters in newly diagnosed and long-standing diabetes patients may be necessary. Therapeutic monoclonal antibodies typically act through two general mechanisms: i) depleting target cell populations and ii) blocking cellular receptor function.
Anti-CD3 antibody: CD3 (Cluster of Differentiation 3) is a protein complex and T cell co-receptor involved in the activation of cytotoxic T cells and T helper cells. The most successful clinical immunotherapy for T1D to date has been the use of anti-CD3 mAb. In 1994, Chatenoud and Bach demonstrated that anti-CD3 therapy could reverse new-onset diabetes in NOD mice and establish long-term remission and β-cell specific tolerance. The protective mechanisms induced by anti-CD3 mAb therapy has been extensively studied in mice. Meanwhile, patients receiving anti-CD3 mAb treatment showed a significant decrease in peripheral T cells and a rebound within a month after treatment. This reduction in numbers is partially believed to be due to T cell existing the circulation. Experimental evidence also suggests that anti-CD3 impacts the T cell phenotype of treated T1D subjects.
Anti-CD20 antibody: CD20 is a membrane-embedded surface molecule involved in B cell development and differentiation into plasma cells. Studies indicates that B cells are the critical driver of human T1D progress. For instance, the expression of CD20-positive B cells is associated with invasive, early-onset diabetes and extensive islet infiltration. A mouse-human chimeric IgG1 mAb specific to human CD20 has been studied in patients with recent onset of T1D. One year after treatment, this anti-CD20 monoclonal antibody-treated subjects showed improvements in HbA1c and C-peptide levels compared to the control group, indicating the preservation of β-cell function.
Anti-CD2 antibody: CD2 is a surface adhesion molecule expressed by multiple cell populations, including T cells, NK cells, and DC cells. A fusion protein, composed of the CD2-binding domain of LFA3 and the human IgG1 Fc region, can preferentially bind to Tmem and induces apoptosis by binding to FcRγ expressed on NK cells via IgG1 Fc. Thus, individuals receiving related therapy experience reduced Tmem levels. Regarding T1D, related clinical trials demonstrate a reduced frequency of activated T cells in the blood and an increased ratio of Tregs to Tmem. Concurrently, evidence from related research suggests that this therapy can prolong β-cell function.
The ultimate goal of T1D immunotherapy is to suppress ongoing β-cell autoimmunity by restoring peripheral tolerance without affecting protective immunity and preserving β-cell function. The complexity of disease process is marked by a variety of immune effectors. However, the different dynamics of disease progression among individuals make it challenging to develop effective immunotherapy so far.
In general, monoclonal antibody therapy has been used to change disease progression by deleting immune effector cells, changing the phenotype / function of effector cells, or blocking soluble/cell surface protein interactions. Clinical therapies targeting T cells and B cells through anti-CD3 and anti-CD20, respectively, has been proved to be safe and effective in maintaining the quality of β cells in newly diagnosed patients. However, these and other treatments have failed to restore self-tolerance in the long term.
An essential variable affecting the efficacy of mAb in T1D is the timing of intervention related to disease progression. The diabetic response in human T1D can be regarded as a series of stages characterized by: 1) the initiation of autoimmune responses detected through the presentation of various islet autoantibodies, 2) sustained autoimmunity with metabolic abnormalities indicating abnormal stress on β-cell group, and 3) obvious diabetic onset, indicating significant loss of β-cell functionality. Most clinical trials have been applied intervention measures during the second and third stages of disease progression. Preclinical studies have made it clear that specific mAb therapy may be effective only at certain stages of T1D progression. Recent clinical results have shown that the efficacy of anti-CD3 therapy in delaying diabetes onset depends on sustained autoimmunity, further emphasizing this key aspect of T1D immunotherapy. Given the complexity of the diabetic response and the parameters associated with effective tolerance to immune effectors, it is likely that monoclonal antibody combinations are needed to target multiple cell types and/or effector molecules. However, regardless of the monoclonal antibody strategy, the ability to restore persistent peripheral tolerance to prevent further destruction of β cells is critical to clinical success.
So far, monoclonal antibody therapy has achieved promising results in influencing Type 1 Diabetes progression. Nevertheless, an effective strategy is needed to rebuild self-tolerance over a long period of time. Ongoing T1D studies continue to characterize new genes and potential targets related to the susceptibility and progression of T1D disease. The ability to customize mAb targets and corresponding effector functions provides great flexibility for the discovery and development of successful T1D mAb treatments.
References
- Ke, Q. I., et al. "Evolving antibody therapies for the treatment of type 1 diabetes." Frontiers in Immunology 11 (2021): 624568.
- Binder, Christian, et al. "CD2 immunobiology." Frontiers in immunology 11 (2020): 1090.
- Distributed under Open Access license CC BY 4.0, without modification.
