Evolving Antibody Therapy for Breast Cancer
Breast cancer, a malignancy driven by the combined influence of genetic and environmental factors, arises from the dysregulated proliferation and apoptosis evasion of mammary epithelial cells due to accumulated genetic alterations. Key mechanisms involve the disruption of critical signaling pathways (e.g., HER2 activation, aberrant estrogen/progesterone receptor signaling), inactivation of tumor suppressor genes (e.g., TP53, BRCA1/2), and activation of oncogenes (e.g., PIK3CA), accompanied by genomic instability (e.g., chromosomal aberrations, microsatellite instability), ultimately fostering malignant transformation. Early-stage (I-II) breast cancer achieves 5-year survival rates exceeding 90% through surgery and adjuvant therapies (radiotherapy, chemotherapy, endocrine therapy), whereas advanced-stage disease necessitates multimodal interventions (e.g., CDK4/6 inhibitor plus endocrine therapy, PARP inhibitors, PD-1/PD-L1 immune checkpoint inhibitors) to prolong survival. Despite the incurable nature of metastatic breast cancer, targeted agents (e.g., specific antibodies) enable sustained survival in some subtypes (e.g., HER2+).
Antibody Therapies in Breast Cancer
Antibody therapies have become a vital strategy in breast cancer management, leveraging their inherent specificity and versatility to target neoplastic cells while mitigating damage to healthy tissue. This approach offers several key advantages over conventional treatments: the capacity to (1) inhibit specific signaling pathways promoting cancer cell growth and proliferation, (2) augment endogenous anti-tumor immune responses by recruiting effector cells, and (3) facilitate targeted delivery of cytotoxic agents via antibody-drug conjugates (ADCs), thereby maximizing efficacy and minimizing systemic toxicity. These diverse mechanisms render antibody therapies a crucial component of contemporary breast cancer care, with the potential to enhance therapeutic outcomes and reduce adverse effects.
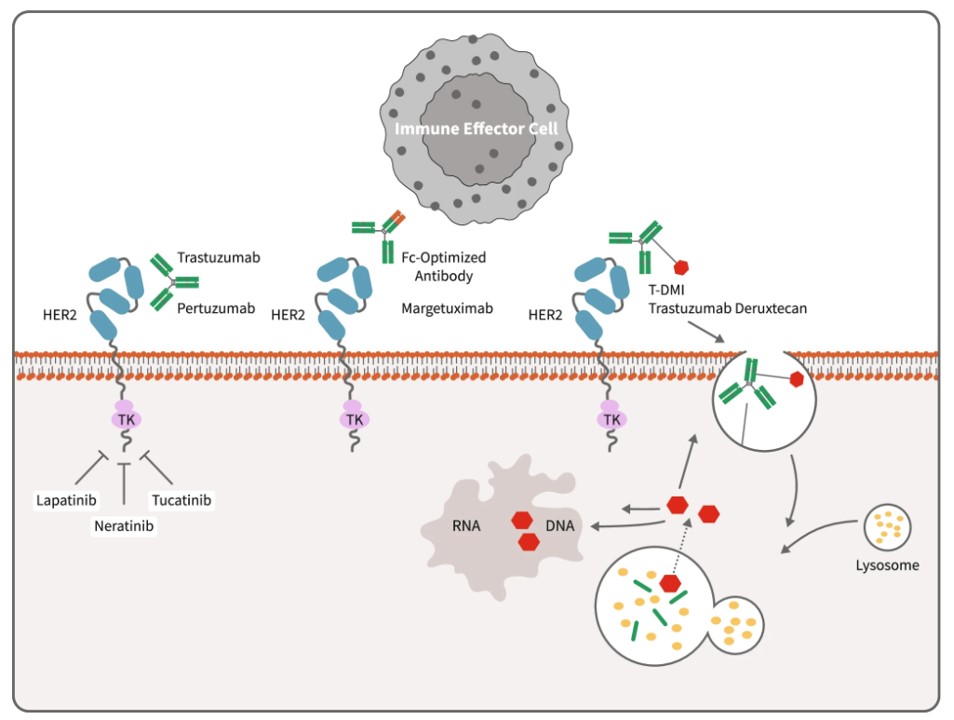 Fig.1 Mechanisms of anti-HER2 therapies.1
Fig.1 Mechanisms of anti-HER2 therapies.1
Monoclonal Antibodies (mAbs)
-
Human Epidermal Growth Factor Receptor 2 (HER2)-Targeting
- Trastuzumab: This mAb targets HER2, a transmembrane tyrosine kinase receptor protein that is overexpressed in approximately 15-20% of breast cancers. Trastuzumab binds to the extracellular domain of HER2, primarily inhibiting the HER2 signaling pathway, which is involved in cell proliferation and survival. Trastuzumab is employed in HER2-positive breast cancer treatment across early and advanced stages, frequently in combination with chemotherapy.
- Pertuzumab: This antibody also targets HER2, binding a distinct epitope within the extracellular domain, specifically domain II. Pertuzumab prevents HER2 from forming heterodimers with other HER family members, such as HER3, which further inhibits downstream signaling and tumor growth. It is used in combination with trastuzumab and chemotherapy in the neoadjuvant and adjuvant settings for HER2-positive breast cancer, as well as in metastatic disease.
- Margetuximab: This is an Fc-engineered, anti-HER2 mAb. Margetuximab targets the same antigen as trastuzumab (HER2), but its Fc region has been modified to enhance binding to activating FcγRIIIa receptors on immune cells, leading to increased antibody-dependent cellular cytotoxicity (ADCC). Margetuximab is approved in a chemotherapy combination regimen for metastatic HER2-positive breast cancer in patients with prior anti-HER2 exposure.
-
Vascular Endothelial Growth Factor (VEGF)-Targeting
- Bevacizumab: This humanized mAb targets VEGF-A, a crucial mediator of angiogenesis. Bevacizumab binds to VEGF-A, preventing it from interacting with its receptors (VEGFR-1 and VEGFR-2) on endothelial cells, thereby inhibiting the formation of new blood vessels that tumors need to grow and metastasize. Its use in breast cancer is more limited, and while it has shown some benefit in certain metastatic settings, overall survival benefits have been modest.
-
Prolactin Receptor (PRLR)-Targeting
- LFA102: This antibody targets PRLR, specifically binding to its extramembrane structural domain to block PRL-induced dimerization and JAK2/STAT5 signaling. PRLR, a receptor involved in cell growth, survival, and migration, is implicated in both breast and prostate cancer cells. The binding of LFA102 to PRLR blocks the interaction of PRL and PL with their receptors, thereby suppressing downstream pro-tumorigenic signaling and promoting tumor cell death via ADCC.
Bispecific Antibodies (BsAbs)
-
HER2/HER3-Targeting
- Zanidatamab (MCLA-128): This BsAb is designed to simultaneously bind to HER2 and HER3, two receptor tyrosine kinases that are frequently overexpressed in breast cancer. By targeting both receptors, Zanidatamab blocks HER2/HER3 heterodimerization, a key mechanism of resistance to HER2-targeted therapies, and inhibits downstream signaling pathways involved in tumor growth. It is currently being evaluated in clinical trials for HER2-expressing breast cancers.
-
HER2-Epitope Targeting
- Zanidatamab (ZW25): This BsAb targets two non-overlapping HER2 epitopes (subdomains II and IV). This unique binding mechanism leads to enhanced HER2 receptor internalization and degradation, effectively removing HER2 from the cell surface and disrupting HER2 signaling. ZW25 is under clinical investigation for the treatment of HER2-positive breast cancer.
While single-agent antibody therapies have demonstrated success, ongoing research is exploring combination regimens, such as trastuzumab and pertuzumab combined with small molecule drugs. These efforts pave the way for further improvements in patient outcomes and a promising future for breast cancer treatment.
Reference
- Wynn, Carrie S., and Shou-Ching Tang. "Anti-HER2 therapy in metastatic breast cancer: many choices and future directions." Cancer and Metastasis Reviews 41.1 (2022): 193-209. Distributed under Open Access license CC BY 4.0, without modification.


