Immunohistochemistry (IHC)
Immunohistochemistry (IHC) is a powerful technique that utilizes the specific binding between the antigen and antibody to detect and localize specific antigens in cells and tissue, often visualized by light microscopy. IHC is widely used in clinical diagnostics in anatomic pathology with the advent of antigen retrieval methods allowing it to be performed conveniently on formalin fixed paraffin embedded (FFPE) tissue and automated methods for high volume processing with reproducibility. Besides, IHC is frequently utilized to assist in the classification of neoplasms, determination of a metastatic tumor’s site of origin and detection of tiny foci of tumor cells inconspicuous on routine hematoxylin and eosin (H&E) staining. Furthermore, IHC is increasingly being used to provide predictive and prognostic information.
General Steps
Steps in IHC can be summarized as follows: tissue fixation, embedding and sectioning, antigen retrieval, permeabilization, blocking, antibody incubation and immunostaining.
- Tissue fixation, embedding and sectioning
- Cells / cytological preparations: 4% formaldehyde
- Tissue sections: 10% Neutral-Buffered Formalin (NBF)
- Antigen Retrieval
- In the enzymatic method, proteases such as proteinase K, trypsin, and pepsin are used.
- The heat-induced method uses heat and a selection of buffers.
- Permeabilization
- Blocking
- Protein Blocking
- Biotin Blocking
- Endogenous Enzyme Blocking
- Immunostaining
Fixation of the tissue sample is vital to maintain cell and tissue morphology during the IHC experiment and storage. It prevents the autolysis and necrosis of excised tissues, preserves antigenicity and enhances the refractive index of tissue constituents. The fixative used is influenced by the target antigen as well as the desired detection technique (fluorescent or chromogenic). The tissue sample is then either embedded in paraffin or frozen. Embedding is important in preserving tissue morphology and giving the tissue support during sectioning. Some epitopes may not survive harsh fixation or embedding. The tissue is typically cut into thin sections or smaller pieces to facilitate further study.
Guidelines for Choosing a Fixative
| Antigen | Fixative |
| Most proteins, peptides and enzymes of low molecular weight |
|
| Delicate tissue | Bouin's fixative |
| Small molecules such as amino acids | 4% formaldehyde |
| Blood-forming organs (liver, spleen, bone marrow) | Zenker’s solution |
| Connective tissue | Helly's solution |
| Nucleic acids | Carnoy’s solution |
| Large protein antigens such as immunoglobulin | Ice-cold acetone or methanol (100%) |
| Nuclear morphology | Zinc formalin |
| For electron microscopy | 4% formaldehyde - 1% glutaraldehyde |
The process of sample fixation can lead to protein cross-linking, which masks antigens and can restrict antigen-antibody binding. Antigen retrieval enables an antibody to access the target protein within the tissue. Masked epitopes could be recovered using either enzymatic/proteolytic antigen retrieval, or heat-induced antigen retrieval methods. The optimal antigen retrieval technique is dependent on the antigen, the tissue, the fixation method and/or primary antibody. Some antigens require a combination of heating and enzyme digestion.
| Buffers for Heat-induced Antigen Retrieval | Enzyme Formulation for Enzymatic Antigen Retrieval |
| Citrate buffer pH 6.0 | Pepsin |
| EDTA buffer pH 8.0 | Proteinase K |
| Tris buffer pH 10.0 | Trypsin |
| Tris-EDTA buffer pH 9.0 |
Permeabilization is required when the antibody needs access to the inside of cells in order to detect the target antigen, such as intracellular proteins and cytoplasmic epitopes of transmembrane proteins. Solvents or detergents are typically used for permeabilization.
Solvent and Detergent Guidelines
| Solvents | Acetone | Acetone fixation will also permeabilize |
| Methanol | Methanol fixation can be used to permeabilize but is not always effective | |
| Detergents | Triton X-100 or NP-40 | Use 0.1 to 0.2% in PBS for 10 min only |
| Polysorbate 20, saponin, digitonin and Leucoperm | Use 0.2 to 0.5% for 10 to 30 min |
IHC often contain one or more blocking steps to reduce background signal and false positives. These steps include: protein blocking, biotin blocking, endogenous enzyme blocking.
Blocking with sera or a protein blocking reagent prevents non-specific binding of antibodies to tissue or Fc receptors. Serum is a common blocking agent as it contains antibodies that bind to non-specific sites. Using a serum matching the species of the secondary antibody is recommended. Proteins such as BSA or casein may also be used to block non-specific antibody binding, and with these there is no need to match the reagent to the species of the secondary antibody.
If a biotin-based detection system is used, blocking endogenous biotin is recommended. Biotin is present in many tissues, particularly in kidney, liver and brain. It is blocked by pre-incubation of the tissue with avidin, followed by incubation with biotin to block additional biotin binding sites on the avidin molecule.
Chromogenic detection methods usually use an enzyme linked to a secondary antibody to visualize antibody localization. If the enzyme is naturally present in the tissue being studied, its activity must be blocked before the detection step. To check for endogenous peroxidase activity, tissues can be incubated with DAB substrate prior to primary antibody incubation. If tissues turn brown, endogenous peroxidase is present and a blocking step is required. A 10-15 minutes incubation in 0.3% hydrogen peroxide is usually sufficient blocking. Tissue can be tested for endogenous alkaline phosphatase (AP) by incubating with BCIP/NBT. If a blue color is observed, endogenous AP is present and blocking is necessary. Levamisole is used for blocking and is added with the chromogenic substrate. Intestinal AP is blocked with a weak acid before adding the primary antibody.
Direct vs Indirect Detection in IHC
In direct detection methods, the primary antibody is directly conjugated to a label. For indirect detection, the primary antibody is bound by a labeled secondary antibody that has been raised against the host species of the primary antibody. Indirect methods may also include amplification steps to increase signal intensity. The choice of direct or indirect detection is often prescribed by the expression level of the target antigen.
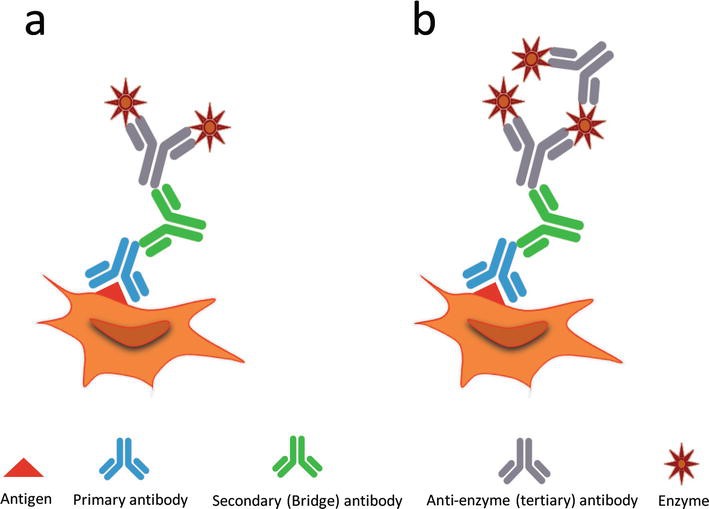 Fig.2 Immunostaining methods.1
Fig.2 Immunostaining methods.1
| Direct Detection | Indirect Detection | |
| Advantages |
|
|
| Disadvantages |
|
|
Chromogenic Detection vs Fluorescent Detection in IHC
| Advantages | Disadvantages | |
| Fluorescent Detection |
|
|
| Chromogenic Detection |
|
|
Summary of Signal Amplification Methods
| Complex Formed | Advantages | |
| Avidin-Biotin Complex (ABC) Method | Avidin-biotin-enzyme | Greater sensitivity than traditional indirect detection. |
| Labeled Streptavidin-Biotin (LSAB) Method | Streptavidin-enzyme |
|
| Polymer-Based Method | Polymer backbone- enzyme |
|
IHC is a complex experiment with multiple parameters that need to be considered and optimized. Our Troubleshooting of Immunohistochemistry (IHC) illustrates the possible sources of common problems and how to combat them. Please refer to the following corresponding section for details.
Related Sections
- Protocol of Immunohistochemistry (IHC)
- Preparation and Chromogenic IHC Staining of Frozen Tissue Sections
- Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections
- Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
Reference
- Shojaeian, Sorour, Nasim Maslehat Lay, and Amir-Hassan Zarnani. "Detection systems in immunohistochemistry." Immunohistochemistry-The Ageless Biotechnology. London: IntechOpen, 2018.


