Immunotherapy Approaches for Breast Cancer
Breast cancer was once considered weakly immunogenic, but recent studies have shown that it has unique immunological characteristics, providing a theoretical basis for immunotherapy. Notably, genomic analyses indicate that breast cancer, particularly triple-negative breast cancer (TNBC), exhibits a high tumor mutation burden (TMB) and significant neoantigen potential, facilitating enhanced immune recognition. Furthermore, approximately 20% of TNBC patients display elevated programmed cell death ligand 1 (PD-L1) expression within tumor cells or the microenvironment, which correlates positively with tumor-infiltrating lymphocyte (TIL) density, suggesting that targeting the dynamic balance between immunosuppression and activation could yield therapeutic benefits. Although immunotherapy adoption in breast cancer trails that of hematologic malignancies, clinical trials with immune checkpoint inhibitors (ICIs) have yielded significant advances, particularly in TNBC, with neoadjuvant approaches using ICI monotherapy or combinations with chemotherapy demonstrating promise for improving patient outcomes.
Immune Checkpoints in Breast Cancer Management
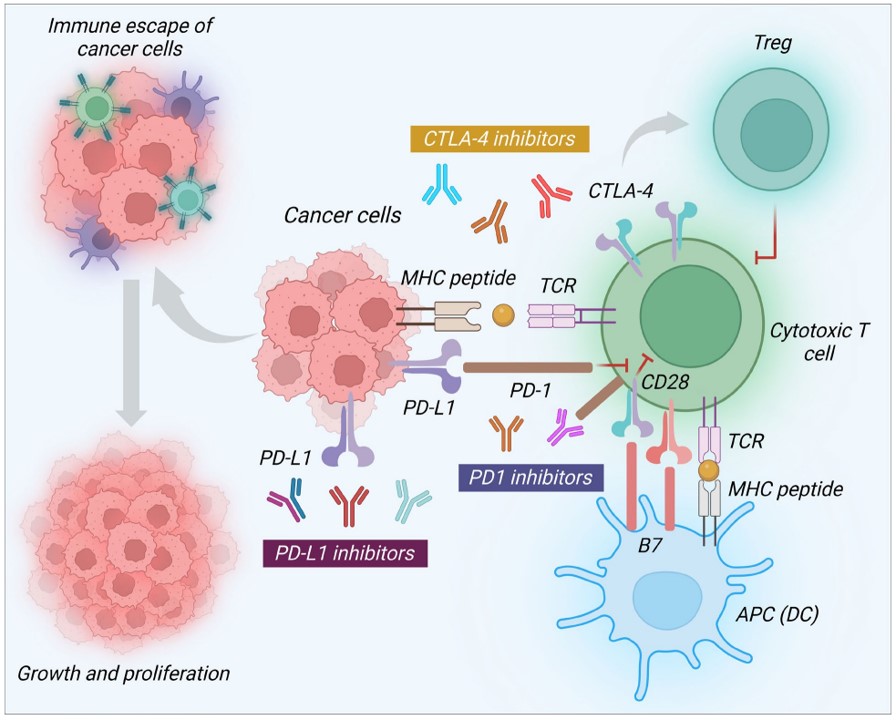 Fig.1 Therapeutic role of ICIs in breast cancer treatment.1,3
Fig.1 Therapeutic role of ICIs in breast cancer treatment.1,3
- PD-1/PD-L1
PD-1 is a co-inhibitory receptor expressed on T cells, B cells, monocytes, and NK cells, with two ligands, PD-L1 and PD-L2, expressed on antigen-presenting cells. The binding of PD-L1 or PD-L2 to PD-1 can downregulate the expression of anti-apoptotic molecules and weaken T-cell activity. PD-1 inhibitors block their interaction with PD-L1 and PD-L2, thereby inhibiting tumor growth. The blockade of the PD-1/PD-L1 axis is considered a potential therapeutic strategy for TNBC. However, early monotherapy studies showed limitations. A phase I trial in advanced pancreatic cancer did not observe significant anti-tumor activity in 14 patients treated with PD-L1 blockade. Preclinical data from murine transplant models, and subsequent clinical trials, have demonstrated that PD-1/PD-L1 blockade combined with chemotherapy enhances anti-tumor effects, indicating that a combination strategy may be more promising than PD-1/PD-L1 inhibition alone.
- Cytotoxic T Lymphocyte Antigen-4 (CTLA-4)
CTLA-4 functions as a co-inhibitory receptor on T cells, while CD28 acts as a co-stimulatory receptor on activated CD4+ and CD8+ T cells. CTLA-4 weakens T cell activity by competing with CD28 to bind B7-1 (also known as CD80) or B7-2 (also known as CD86) ligands on antigen-presenting cells and transmitting inhibitory signals to T cells. Blocking CTLA-4 can induce anti-tumor activity. Furthermore, CTLA-4 binding also blocks IL-2 transcription, which is essential for T-cell growth and NK-based cytotoxicity. Therefore, blocking CTLA-4 with monoclonal antibodies can increase the effector function of T cells in killing cancer cells.
- Lymphocyte activation gene-3 (LAG-3)
LAG-3, a recently identified immune checkpoint, is predominantly expressed on regulatory T cells (Treg) and functionally exhausted effector T cells. By binding to MHC class II molecules, LAG-3 delivers inhibitory signals, attenuating CD8+ T cell anti-tumor activity while simultaneously enhancing Treg-mediated immunosuppression. Anti-LAG-3 antibodies can effectively restore the proliferative capacity and cytokine (such as IFN-γ, TNF-α) secretion of CD8+ T cells by blocking LAG-3/MHC II interaction, thereby reshaping the tumor immune microenvironment. Although research on LAG-3 inhibitors in breast cancer is still in its early stages, preliminary clinical data show potential. A phase I/II trial in 30 patients with advanced breast cancer who received paclitaxel combined with anti-LAG-3 antibody showed a significant increase in the objective response rate (ORR), suggesting that synergistic chemotherapy can overcome the limitations of monotherapy response. Preclinical studies further indicate that co-inhibition of the LAG-3 and PD-1 pathways is an important mechanism of immune resistance, and dual-target blockade (such as LAG-3×PD-1 bispecific antibodies) mediates stronger T cell activation and tumor regression in breast cancer models.
- T cell immunoglobulin domain and mucin domain-3 (TIM-3)
TIM-3, also designated CD366 or HAVCR2, functions as a negative regulatory immune checkpoint protein. Ligation of TIM-3 by ligands (e.g., galectin-9, HMGB1) elicits T-cell exhaustion and enhances the immunosuppressive activity of myeloid-derived suppressor cells (MDSCs). In TNBC, TIM-3 exhibits elevated expression and co-localizes with PD-1 on exhausted T cells. Preclinical studies indicate that combined TIM-3 and PD-1 blockade amplifies MAGE-A11-specific cytotoxic T lymphocyte (CTL) cytotoxicity against breast cancer cells, suggesting the therapeutic potential of this dual blockade strategy.
Adoptive T-cell therapy
Chimeric Antigen Receptor (CAR) T-cell therapy is a recent and potent form of adoptive T-cell therapy. It leverages the precise specificity of antibodies to direct cytotoxic T cells against designated tumor-associated antigens, exhibiting significant potential in the treatment of breast cancer (including the challenging triple-negative subtype). Preclinical studies have identified a diverse array of target antigens such as EGFR, FRα, NKG2D, HER2, AXL, c-Met, integrin αvβ3, MUC1, ROR1, mesothelin, TROP2, and TEM8. These target antigens have shown effective targeting in both in vitro and in vivo models. However, the transition to clinical application has revealed a complex interaction between therapeutic efficacy and severe toxicity. Early-phase trials targeting antigens such as c-Met and MUC1 have shown mixed outcomes. Some trials have demonstrated acceptable safety profiles and evidence of antitumor activity, while others have been terminated or suspended due to unacceptable adverse events. These adverse events are often attributed to off-target effects arising from the co-occurrence of antigen expression between tumors and healthy tissues, underscoring the critical need for strategies to enhance tumor-specific targeting. In response to these challenges, the investigation of tumor-specific glycoforms of tumor-associated antigens, characterized by aberrant glycosylation patterns resulting from dysregulated cellular machinery, presents a promising avenue for refining CAR T-cell therapies. Because these glycoforms (including Tn, T, and sialyl-Tn) exhibit relatively restricted expression in tumor cells, they offer the potential to develop CAR T-cells with enhanced selectivity, which may mitigate off-target toxicity and improve the therapeutic index of this immunotherapeutic approach.
References
- Ye, Feng, et al. "Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer." Molecular cancer 22.1 (2023): 105.
- Dvir, Kathrin, Sara Giordano, and Jose Pablo Leone. "Immunotherapy in breast cancer." International Journal of Molecular Sciences 25.14 (2024): 7517.
- Distributed under Open Access license CC BY 4.0, without modification.


