GITR Antibodies
Background
GITR (glucocorticoid-induced tumor necrosis factor receptor-associated protein) is an important signaling protein existing on the cell membrane surface, mainly expressed on the surface of activated T lymphocytes and regulatory T cells. This protein can bidirectionally regulate the activation and inhibition signals of T cells by binding to specific ligands, thereby playing a key role in immune responses. In tumor immunotherapy research, agonists targeting GITR have been widely explored for relieving immunosuppression in the tumor microenvironment. This gene was first identified and named by the team of Japanese scientist Sadao Ikemoto in 1997 when they were studying the effect of glucocorticoids on T cells. As a member of the tumor necrosis factor receptor superfamily, the precise three-dimensional structure and signal transduction mechanism of GITR have been deeply analyzed, providing a key theoretical basis for the development of new immunomodulatory drugs and greatly promoting the development of immunology and tumor treatment fields.
Structure of GITR
GITR is a type I transmembrane protein with a molecular weight of approximately 45 kDa. The molecular weight of this protein varies slightly among different species due to differences in the degree of glycosylation and amino acid sequence.
| Species | Human | Mouse | Rat | Crab-eating macaque | Dog |
| Molecular Weight (kDa) | 16.7 | 16.9 | 16.8 | 16.5 | 16.7 |
| Primary Structural Differences | Contains three cysteine-rich domains | High homology with human and conserved ligand-binding domains | Extracellular region structure similar to height in mice | Similar to human GITR functions, often used in preclinical studies | The core structure of signal transduction domain conservative |
The GITR protein is composed of 241 amino acids, and its extracellular region forms a typical tumor necrosis factor receptor-like folded conformation. This protein structure contains three characteristic cysteine-rich domains, which are stabilized by intramolecular disulfide bonds to form specific ligand binding interfaces. The trimerization of GITR is the structural basis for its interaction with the ligand GITRL. The secondary structure of this protein is mainly composed of β -sheets, which are arranged in a conserved "Greek key" topological pattern. The key F/G loop regions are directly involved in ligand recognition and binding processes, while the domains near the membrane are responsible for the initiation of signal transduction. This ingenious structural design enables GITR to precisely regulate the activation of T cells and immune responses.
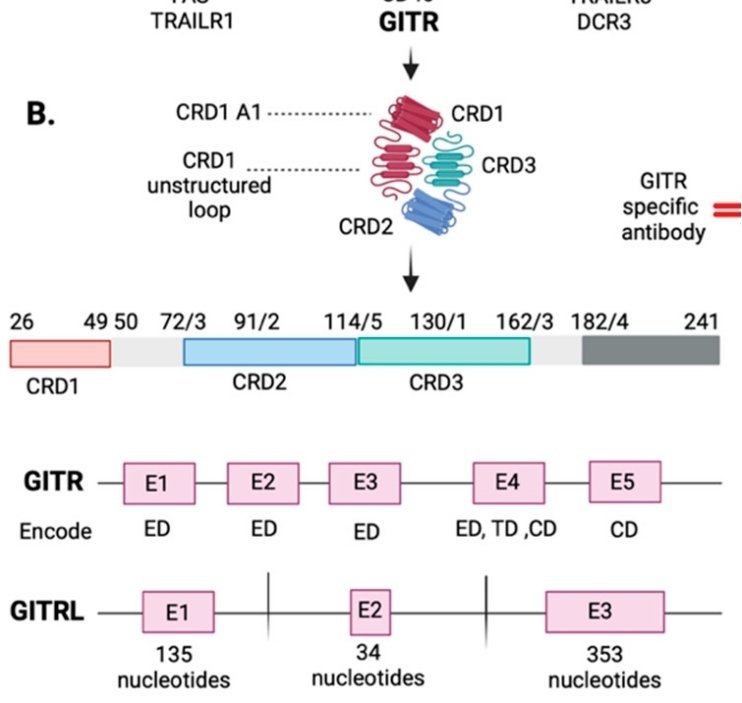 Fig. 1 GITR and GITRL genes' structure in exons.1
Fig. 1 GITR and GITRL genes' structure in exons.1
Key structural properties of GITR:
- A typical TNFR superfamily folding configuration
- Conservative CRD functional areas
- Specific ligand binding motif sequence
- Intracellular signal transduction module
Functions of GITR
The main function of GITR is to act as a co-stimulatory signal regulator for T cell activation. However, this receptor is also involved in multiple immunomodulatory processes, including the inhibition of regulatory T cell function and the enhancement of effector T cell activity.
| Function | Description |
| T-cell co-stimulation | By activating the NF-κB and MAPK signaling pathways, it enhances T cell receptor-mediated proliferation and cytokine production. |
| Regulatory T cell function regulation | Weaken the immunosuppressive function of Treg cells while enhancing the resistance of effector T cells to them. |
| Maintenance of immune homeostasis | In autoimmunity and tumor immunity play a two-way adjustment function, balance the strength of the immune response and duration. |
| Cell survival promotion | By up-regulating the expression of anti-apoptotic proteins, the survival time of effector T cells is prolonged and immune memory is enhanced. |
| Tumor immune surveillance | In the tumor microenvironment, by breaking the immunosuppressive state, the tumor-killing ability of CD8+ T cells is enhanced. |
The functional mechanism of GITR differs from that of traditional costimulatory molecules. It plays a unique "immune balancer" role in the immune response by bidirectionally regulating the balance between effector T cells and regulatory T cells. This characteristic makes it an important target for tumor immunotherapy.
Applications of GITR and GITR Antibody in Literature
1. Tian, Jie, et al. "The role of GITR/GITRL interaction in autoimmune diseases." Frontiers in immunology 11 (2020): 588682. https://doi.org/10.3389/fimmu.2020.588682
The article indicates that GITR is a member of the tumor necrosis factor receptor family and is widely expressed in immune cells such as T cells. After binding to the ligand GITRL, it can regulate the functions of effector T cells, regulatory T cells and myeloid cells, and participate in the occurrence and development process of autoimmune diseases, tumors and inflammatory diseases.
2. Sathe, Anuja, et al. "GITR and TIGIT immunotherapy provokes divergent multicellular responses in the tumor microenvironment of gastrointestinal cancers." Genome Medicine 15.1 (2023): 100. https://doi.org/10.1186/s13073-023-01259-3
In this study, using patient-derived tumor section models, it was found that the effect of GITR agonists is limited. They can only enhance the expression of effector genes in cytotoxic CD8 T cells, but have no effect on functionally exhausted CD8 T cells. This indicates that its activation ability in the tumor microenvironment has limitations.
3. Papadakos, Stavros P., et al. "Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma." Cancers 16.14 (2024): 2609. https://doi.org/10.3390/cancers16142609
The article indicates that GITR, as a new target for immunotherapy, has attracted much attention in tumors such as hepatocellular carcinoma. It exerts anti-tumor effects by activating effector T cells and inhibiting Treg function, especially showing potential when combined with PD-1 inhibitors, providing new therapeutic possibilities for patients in the advanced stage.
4. Krausz, Ludovic Tibor, et al. "GITR‐GITRL system, a novel player in shock and inflammation." The Scientific World Journal 7.1 (2007): 533-566. https://doi.org/10.1100/tsw.2007.106
The article indicates that GITR exerts pro-inflammatory effects in inflammation and shock by activating neutrophils, macrophages and effector T cells, and inhibiting the function of regulatory T cells. This article reviews the in vivo mechanism of action and therapeutic potential of the GITR/GITRL system in related diseases.
5. Ronchetti, Simona, et al. "CD8+ T cells: GITR matters." The Scientific World Journal 2012.1 (2012): 308265. https://doi.org/10.1100/2012/308265
The article indicates that GITR is a member of the TNF receptor family and plays a key role in immune cells such as CD8+ T cells. Studies have shown that GITR plays a significant regulatory role in antiviral, anti-tumor and various autoimmune disease models by promoting CD8+ T cell responses.
Creative Biolabs: GITR Antibodies for Research
Creative Biolabs specializes in the production of high-quality GITR antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom GITR Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our GITR antibodies, custom preparations, or technical support, contact us at email.
Reference
- Papadakos, Stavros P., et al. "Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma." Cancers 16.14 (2024): 2609. https://doi.org/10.3390/cancers16142609
Anti-GITR antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Mouse Anti-EMP3 Recombinant Antibody (CBFYE-0100) (CBMAB-E0207-FY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-COL12A1 Recombinant Antibody (CBYY-C3117) (CBMAB-C4560-YY)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Mouse Anti-ASB9 Recombinant Antibody (1D8) (CBMAB-A0529-LY)

-
Mouse Anti-CARD11 Recombinant Antibody (CBFYC-0811) (CBMAB-C0866-FY)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Mouse Anti-ENO1 Recombinant Antibody (8G8) (CBMAB-E1329-FY)

-
Mouse Anti-CGAS Recombinant Antibody (CBFYM-0995) (CBMAB-M1146-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








