MCL1 Antibodies
Background
The MCL1 gene encodes an anti-apoptotic protein belonging to the BCL-2 protein family, which mainly functions on the mitochondrial membrane. This protein maintains cell survival by binding to and inhibiting pro-apoptotic proteins and plays a crucial role in the regulation of embryonic development, lymphocyte homeostasis and tissue homeostasis. Its expression level is abnormally elevated in various cancer cells, becoming one of the important mechanisms of tumor drug resistance, and thus has been listed as an important target for cancer treatment. This gene was discovered by the Kozopas team in myeloid leukemia cells in 1993. Its unique short-life characteristic (rapid degradation) and multiple splicing variants provide an important model for the study of cell fate regulation. In-depth research on the structure of the MCL1 protein not only promotes the development of targeted drugs but also deepens people's understanding of the molecular mechanism of apoptosis and its association with diseases.
Structure of MCL1
MCL1 is a protein with a molecular weight of approximately 37.4 kDa. Its actual weight varies depending on different splicing variants, with longer isomers (such as McL1-l) having a larger molecular weight.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | 37.4 (Main variant) | 36.8 | 37.1 |
| Primary Structural Differences | Anti-apoptosis, maintaining cell survival | Is essential in embryonic development | The functions of the human MCL1 are highly conserved |
The MCL1 protein belongs to the BCL-2 family, and its structure contains four BH domains (BH1-BH4) specific to the BCL-2 family. These domains form a hydrophobic pocket through α -helical bundles. The core of this protein lies in its ability to bind to BH3-only proteins (such as BIM and PUMA). A special N-terminal region determines the rate at which it is ubiquitinated and degraded, which endows MCL1 with a unique short-lifetime characteristic to respond rapidly to cellular signals. A key α -helix is directly involved in regulating its conformation, exposing its BH3-binding groove in the "activated" state, thereby performing its anti-apoptotic function.
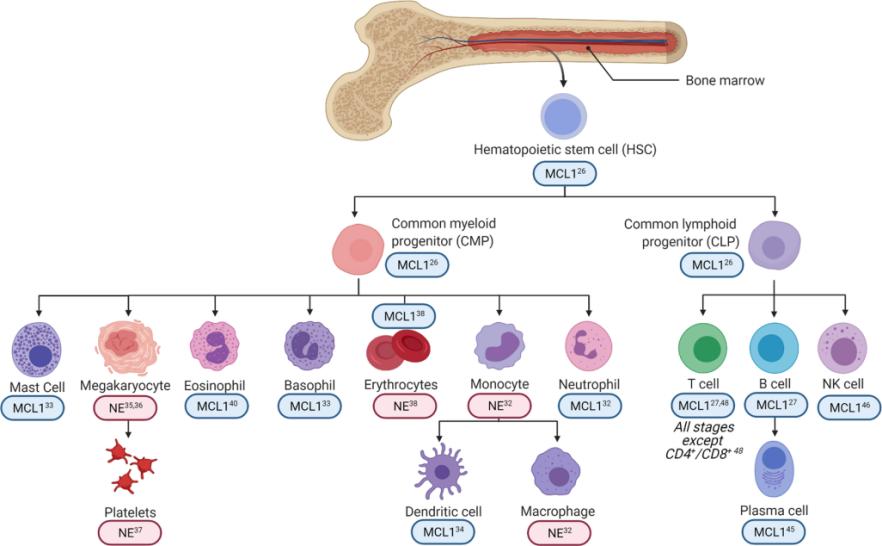 Fig. 1 MCL1 differentially regulates hematopoietic cell survival and differentiation.1
Fig. 1 MCL1 differentially regulates hematopoietic cell survival and differentiation.1
Key structural properties of MCL1:
- Contains BH1, BH2, BH3, BH4 four characteristics of domain structure
- Hydrophobic grooves are formed for binding pro-apoptotic proteins
- Has the unique N control area control protein stability
- Maintain the three-dimensional conformation through the α -helical beam
Functions of MCL1
The core function of the MCL1 gene is to regulate apoptosis to maintain cell survival. However, it is also involved in a variety of cellular processes, including metabolic adaptation, lymphocyte homeostasis and tumorigenesis.
| Function | Description |
| Inhibit apoptosis | In the mitochondrial outer membrane, it binds to pro-apoptotic proteins (such as BIM and BAK) and directly prevents the mitochondrial membrane permeability transition and cytochrome c release. |
| Maintain cell survival | Provide essential survival signals for cells undergoing stress or differentiation signals, such as lymphocytes, hematopoietic stem cells. |
| Developmental support | During the embryonic development stage, it is crucial for the formation and maintenance of various tissues and organs. Its absence can lead to the death of early embryos. |
| Metabolic adaptation | There is a cross-talk with cellular metabolic pathways to regulate cell fate in response to changes in nutritional status. |
| Tumorigenesis promotion | Overexpressed in a variety of cancers, it drives tumor growth and induces chemotherapy resistance by inhibiting cancer cell apoptosis. |
The mechanism of action of MCL1 protein depends on its dynamic binding to pro-apoptotic partners. Its activity is precisely regulated by various post-translational modifications such as phosphorylation and ubiquitination, which enables it to rapidly respond to intracellular and extracellular signals and determine the life and death of cells.
Applications of MCL1 and MCL1 Antibody in Literature
1. Widden, Hayley, et al. "MCL1 binds and negatively regulates the transcriptional function of tumor suppressor p73." Cell Death & Disease 11.11 (2020): 946. https://doi.org/10.1038/s41419-020-03068-7
This study reveals a novel mechanism by which the anti-apoptotic protein MCL1 directly inhibits the transcriptional activity of p73 through its reverse BH3 domain. This localization not only clarifies the function of MCL1 in DNA damage response and cell cycle regulation, but also provides a theoretical basis for the combined application of MCL1 inhibitors and platinum-based chemotherapy.
2. Fu, Dechen, et al. "MCL1 nuclear translocation induces chemoresistance in colorectal carcinoma." Cell Death & Disease 13.1 (2022): 63. https://doi.org/10.1038/s41419-021-04334-y
In p53-deficient colorectal cancer, studies have found that DNA damage chemotherapy can induce nuclear translocation of MCL1, which interacts with proteins such as enolase through its unique loop domain, generating non-classical chemotherapy resistance. This move unexpectedly exposed the dependence of cancer cells on Bcl-xL, providing a new therapeutic strategy for the combined use of chemotherapy and Bcl-xL inhibitors.
3. Pratelli, Giovanni, et al. "MCL1 inhibition overcomes the aggressiveness features of triple-negative breast cancer MDA-MB-231 cells." International journal of molecular sciences 24.13 (2023): 11149. https://doi.org/10.3390/ijms241311149
Research has found that in triple-negative breast cancer, the use of BH3 mimic A-1210477 to inhibit MCL1 not only induces nest-loss apoptosis but also effectively weakens the invasion and metastasis ability of cancer cells. The mechanisms include disrupting adhesion plaque signaling, down-regulating matrix metalloproteinases, and reversing epithelial-mesenchymal transition and dry characteristics, revealing the key role of MCL1 in inhibiting malignant tumor progression.
4. Jacob, Maureen, et al. "Increased MCL1 dependency leads to new applications of BH3-mimetics in drug-resistant neuroblastoma." British journal of cancer 129.10 (2023): 1667-1678. https://doi.org/10.1038/s41416-023-02430-8
Research has found that in chemotherapy-resistant neuroblastoma, survival is highly dependent on the MCL1 protein. Based on this, combining BH3 mimics targeting MCL1 with NK cell-based immunotherapy offers a promising new strategy for the treatment of recurrent neuroblastoma.
5. Duan, Lei, et al. "Novel markers of MCL1 inhibitor sensitivity in triple-negative breast cancer cells." Journal of Biological Chemistry 300.6 (2024). https://doi.org/10.1016/j.jbc.2024.107375
This study identified a predictive marker composed of four genes to address the issue of inconsistent efficacy of MCL1 inhibitors in triple-negative breast cancer. This characteristic spectrum can not only predict the sensitivity of MCL1 inhibitors, but also the small molecule inhibitors in its pathway can overcome drug resistance, providing a new strategy for tumor stratification and combination therapy.
Creative Biolabs: MCL1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality MCL1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MCL1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MCL1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Widden, Hayley, and William J. Placzek. "The multiple mechanisms of MCL1 in the regulation of cell fate." Communications biology 4.1 (2021): 1029. https://doi.org/10.1038/s42003-021-02564-6
Anti-MCL1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Mouse Anti-BACE1 Recombinant Antibody (61-3E7) (CBMAB-1183-CN)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Rabbit Anti-CBL Recombinant Antibody (D4E10) (CBMAB-CP0149-LY)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Mouse Anti-NSUN6 Recombinant Antibody (D-5) (CBMAB-N3674-WJ)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-APP Recombinant Antibody (DE2B4) (CBMAB-1122-CN)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-ESR1 Recombinant Antibody (Y31) (CBMAB-1208-YC)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






