SLC7A11 Antibodies
Background
The SLC7A11 gene encodes the light chain subunit xCT of an important cystine/glutamic acid reverse transporter (system xc-), which is mainly located on the cell membrane. Its function is to mediate the uptake of cystine extracellular and the efflux of glutamic acid intracellular, which is crucial for maintaining the synthesis of glutathione intracellular, the balance of reactive oxygen species (ROS), and the regulation of the ferroptosis process. This gene is often overexpressed in various cancer cells and helps tumor cells resist ferroptosis by enhancing antioxidant capacity. Therefore, it has become a hot target in cancer research. Since its functions were clarified, the regulatory mechanisms and pathophysiological effects of SLC7A11 have been widely studied, greatly enhancing our understanding of cellular oxidative metabolism, tumor adaptation mechanisms, and new forms of programmed cell death.
Structure of SLC7A11
The xCT protein encoded by the SLC7A11 gene is a transmembrane protein of approximately 55 kDa, and its molecular weight may vary depending on the degree of glycosylation modification. This protein contains twelve typical transmembrane domains, forming its unique reverse transporter channels. The core functional domain of the xCT protein is responsible for mediating substrate recognition and transport. Its active center achieves the reverse exchange of one molecule of intracellular glutamic acid and one molecule of extracellular cystine through precise conformational changes. The activity of this protein strictly depends on the CD98hc (SLC3A2) heavy chain protein on the cell membrane. The two are covalently linked through disulfide bonds to form a complete system XC-heterodimer complex. This interaction is crucial for the correct membrane localization and functional maintenance of the protein.
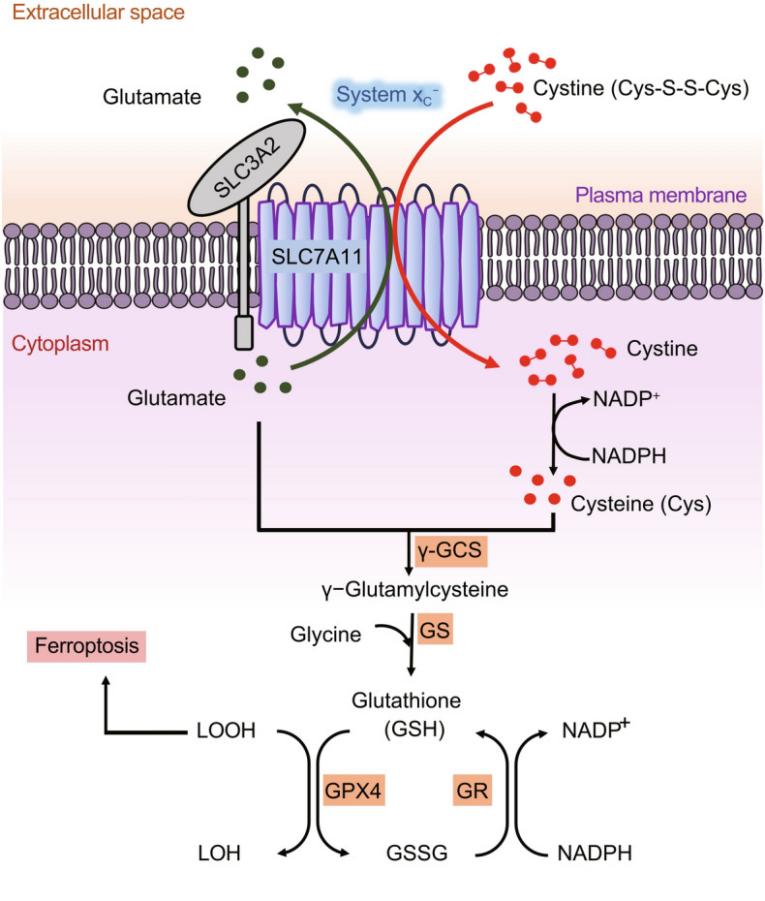 Fig. 1 Structure and function of SLC7A11.1
Fig. 1 Structure and function of SLC7A11.1
Key structural properties of SLC7A11:
- Twelve transmembrane domains
- Conservative substrate bonding pocket
- Dependent on the CD98hc (SLC3A2) subunit
Functions of SLC7A11
The core function of the xCT protein encoded by the SLC7A11 gene is to act as a specific light chain for the system xc⁻, mediating the 1:1 reverse transport of cystine to glutamic acid on the cell membrane. Its specific physiological and pathological functions are as follows:
| Function | Description |
| Cystine uptake | Mediating the uptake of cystine extracellular, which is a key rate-limiting step in the intracellular synthesis of glutathione (GSH). |
| Antioxidant defense | By maintaining GSH levels, it helps maintain the REDOX balance of cells and resist damage caused by reactive oxygen species (ROS). |
| Regulation of ferroptosis | The transport activity is the key to the inhibition of iron death hub, inhibition will directly lead to accumulation of lipid peroxides and induce cell death. |
| Support for tumor progression | In a wide variety of cancer high expression, by enhancing antioxidant capacity to promote tumor growth, and is closely related to the chemotherapy drug resistance. |
| Regulation of neural function | In the brain, it affects excitotoxicity and the survival of neurons by regulating extracellular glutamate levels. |
The kinetic characteristics of the system's function are characterized by high affinity for both cystine and glutamic acid. However, the stoometric ratio of its reverse transport determines that its net effect is to promote antioxidant synthesis, while also potentially increasing the risk of glutamic acid excitotoxicity.
Applications of SLC7A11 and SLC7A11 Antibody in Literature
1. Koppula, Pranavi, Li Zhuang, and Boyi Gan. "Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy." Protein & cell 12.8 (2021): 599-620. https://doi.org/10.1007/s13238-020-00789-5
The article indicates that SLC7A11 is highly expressed in various cancers, promoting tumor development by inhibiting ferroptosis and inducing metabolic reprogramming, while causing cancer cells to rely on glucose and glutamine. This characteristic makes it a potential therapeutic target. This article reviews its regulatory mechanism and related therapeutic strategies.
2. Chen, Qian, et al. "SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma." Cell Death & Differentiation 30.1 (2023): 137-151. https://doi.org/10.1038/s41418-022-01051-7
This study reveals that SOCS2 induces ferroptosis by mediating the ubiquitination degradation of K48-linked SLC7A11, thereby reducing its protein level and ultimately enhancing the radiosensitivity of hepatocellular carcinoma. This indicates that targeting this pathway can enhance the efficacy of radiotherapy.
3. Shen, Liliang, et al. "PHGDH inhibits ferroptosis and promotes malignant progression by upregulating SLC7A11 in bladder cancer." International journal of biological sciences 18.14 (2022): 5459. https://doi.org/10.7150/ijbs.74546
Research has revealed that PHGDH, which is highly expressed in bladder cancer, can bind to PCBP2 and inhibit its degradation. Subsequently, PCBP2 stabilizes the mRNA of SLC7A11, ultimately inhibiting ferroptosis and promoting tumor progression by upregulating SLC7A11. The inhibitor NCT-502 targeting PHGDH has shown therapeutic potential.
4. Yuan, Siyu, et al. "Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF‐1α/SLC7A11 pathway." Cell proliferation 55.1 (2022): e13158. https://doi.org/10.1111/cpr.13158
This study found that sorafenib specifically triggers ferroptosis in hepatic stellate cells by inhibiting HIF-1α and down-regulating the expression of SLC7A11 downstream of it, rather than in hepatocytes or macrophages. This mechanism is the key for it to exert its anti-liver fibrosis effect.
5. Koppula, Pranavi, et al. "Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer." Cancer communications 38.1 (2018): 12. https://doi.org/10.1186/s40880-018-0288-x
This study found that SLC7A11 is overexpressed in cancer and takes up cystine to synthesize glutathione to resist oxidative stress and ferroptosis. Research has found that it also plays a key role in glutamine metabolism and regulates the dependence of cancer cells on glucose and glutamine. Its metabolic function provides a new direction for targeted therapy.
Creative Biolabs: SLC7A11 Antibodies for Research
Creative Biolabs specializes in the production of high-quality SLC7A11 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom SLC7A11 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our SLC7A11 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Koppula, Pranavi, Li Zhuang, and Boyi Gan. "Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy." Protein & cell 12.8 (2021): 599-620. https://doi.org/10.1007/s13238-020-00789-5
Anti-SLC7A11 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Mouse Anti-C5AR1 Recombinant Antibody (R63) (CBMAB-C9553-LY)

-
Mouse Anti-CIITA Recombinant Antibody (CBLC160-LY) (CBMAB-C10987-LY)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-ENO2 Recombinant Antibody (85F11) (CBMAB-0276CQ)

-
Mouse Anti-CCND2 Recombinant Antibody (DCS-3) (CBMAB-G1318-LY)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Mouse Anti-CD19 Recombinant Antibody (CBXC-1224) (CBMAB-C1491-CQ)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

-
Mouse Anti-ADGRE2 Recombinant Antibody (V2-261270) (CBMAB-C0813-LY)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








