Cell Preparation for Flow Cytometry
Flow cytometry is a widely used approach for analyzing the expression of cell surface and intracellular molecules on per cell basis. This technique could be used to characterize and define different cell types in heterogeneous populations, assess the purity of isolated subpopulations, and analyze cell size and volume. The most appropriate samples for flow cytometry are non-adherent cells from tissue cell culture. Solid tissue samples, adherent cell lines as well as tumor samples require further processing before they can be analyzed.
In flow cytometry, the preparation of single cells is extremely important. It requires that samples should be dispersed into single cells, but also that the single cells should maintain their inherent biochemical components and biological characteristics. Many methods are available and may involve enzymatic digestion or mechanical dissociation of the sample. Care should be used when chelation or enzymatic digestion are used, as these may result in the destruction of the antibody epitope. In all situations, removing cell clumps, dead cells, and debris is essential to eliminate false positives and obtain results of the highest quality.
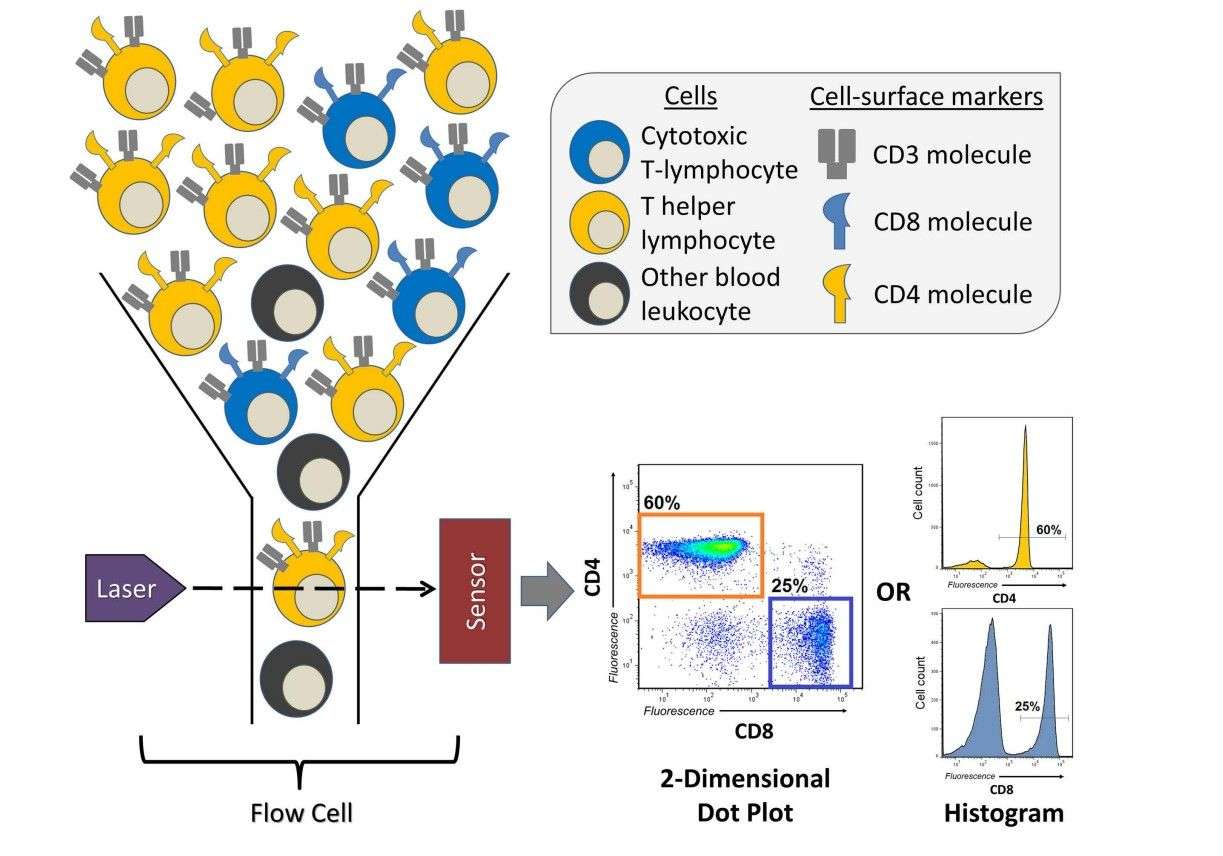 Fig.1 A brief overview of a flow cytometry experiment identifying T cells1.
Fig.1 A brief overview of a flow cytometry experiment identifying T cells1.
Our Protocol of Cell Preparation for Flow Cytometry includes the following parts:
- Protocol A: Preparation of Human Peripheral Blood Mononuclear Cells
- Protocol B: Preparation of Tissue Culture Cells for Flow Cytometry
- Preparation of Cells Stored in Liquid Nitrogen
- Preparation of Tissue Culture Cell Lines in Suspension
- Preparation of Adherent Tissue Culture Cell Lines
- Protocol C: Preparation of Peritoneal Macrophages, Bone Marrow, Thymus and Spleen Cells
The Basic Principles of Flow Cytometry Sample Preparation Are Listed as Follows.
- The cell samples should be fresh. Sample preparation should be finished as soon as possible.
- Appropriate washing, enzyme digestion as well as EDTA treatment should be applied for different cell samples to remove impurities and detach adhesive cells to form single cell suspension.
- Enzyme digestion, mechanical dispersion and chemical dispersion can be applied to tumor tissue to obtain single cell suspension.
- The paraffin embedded tissue should be cut into 40~50 μm thick slices first. After dewaxing to water, the single cell suspension can be prepared with the previous method.
- Single cells must be suspended at an appropriate density to keep the narrow bores of the flow cytometer. The number of single cells in suspension should not be less than 1x106/mL for phenotyping, apoptosis, DNA content, GFP or similar types of experiments.
Our Tips
- Eliminating Dead Cells from Analysis
- Aggregates
- Use nylon mesh to filter samples if aggregates are visibly present
- Use DNAse to eliminate free DNA
- Use a commercial product to keep cells dissociated
- Fixing Cells with Formaldehyde and Increased Autofluorescence
-
When fixing cells for immunofluorescent experiments with formaldehyde, increased autofluorescence is often encountered. The resultant decrease in separation between the negative and positive populations can render some experiments useless. The most common reason for increased autofluorescence is pH drift of the formaldehyde. It is important that correct pH is established in fresh formaldehyde and that pH is monitored as the fixative solution ages.
Dead cells tend to be more auto-fluorescent than live cells, bind antibody non-specifically, and are difficult to completely eliminate from analysis based solely on forward and side scatter. Thus, it is suggested that a fluorescent viability marker be added to most cell preparations before performing flow cytometry.
Cell types such as granulocytes, monocytes, and adherent cell lines tend to form aggregates that will plug the instrument if they're too large. Three methods are available to remove the aggregates or dispersed by some other method before running on the flow cytometer.
Reference
-
Verschoor, Chris P., et al. "An introduction to automated flow cytometry gating tools and their implementation." Frontiers in immunology 6 (2015): 380.
Distributed under Open Access license CC BY 4.0, without modification.
