Immunofluorescence (IF)
Introduction
Immunofluorescence (IF) is a histochemical staining technique used for demonstrating the presence of antibodies bound to antigens in tissues or serum. IF relies on the use of antibodies chemically labeled with fluorescent dyes to visualize molecules under a light microscope. For a successful IF staining, it is important to have a good antibody that will specifically detect the antigen within the molecule of interest. IF permits early diagnosis, treatment as well as subsequent monitoring of some potentially life-threatening disorders. Besides, it is also used in research to advance the understanding and classification of the immunobullous disorders.
Principle
In IF, antigens, antibodies and their complexes are viewed under a fluorescence microscope. The combination of antibody with its specific antigen does not lead to a visible change and thus a readily identifiable label namely fluorochrome must be irreversibly bound to the antibody so that its localization can be recognized.
Fluorescent materials give off light because of their atomic structure. Electrons are arranged in discrete energy levels. When an electron absorbs the energy from a photon of light, it becomes excited and jumps to a higher, less stable energy level. The excited state has a half-life of less than 10 seconds. The electron loses a small amount of energy as heat and the remainder is given off in the form of a photon. The emitted light has a lower energy than the absorbed light, hence has a longer wavelength. The emitted light is almost invariably in the visible spectrum. These fluorochrome markers are detected with a fluorescence microscope equipped with a mercury-vapor or xenon light source, and appropriate exciter and barrier filters. The exciter filter serves to shed light of necessary wavelength on the examined slide, while the barrier filter stops the exciting photons, letting through only the fluorescent light.
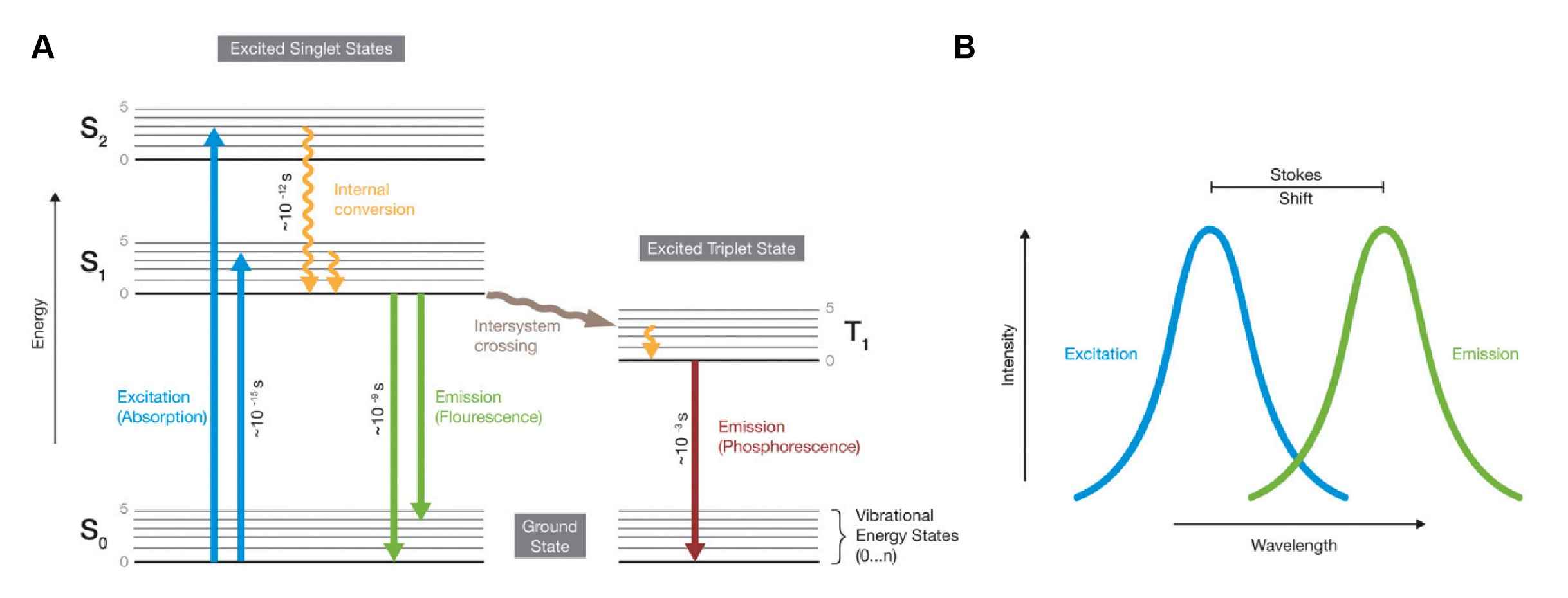 Fig.1 Principle of fluorescence1.
Fig.1 Principle of fluorescence1.
In clinical immunodermatology, there are three basic types of IF techniques: indirect, direct and complement techniques.
Indirect Immunofluorescence (IIF)
IIF utilizes a two-step serological technique to identify circulating autoantibodies in a patient’s serum. In IIF, a primary, unlabeled antibody binds to the target, after which a fluorophore-labeled second antibody is used to detect the primary antibody. IIF is more complicated and time-consuming but it is more sensitive because more than one secondary antibody can bind to each primary antibody, which amplifies the fluorescence signal. A variation of IIF testing is used to detect circulating autoantibodies in immunobullous diseases. In this situation, the primary antibody is the suspected autoantibody in the patient serum. However, IIF is less applicable in the diagnosis of oral manifestations of mucocutaneous diseases.
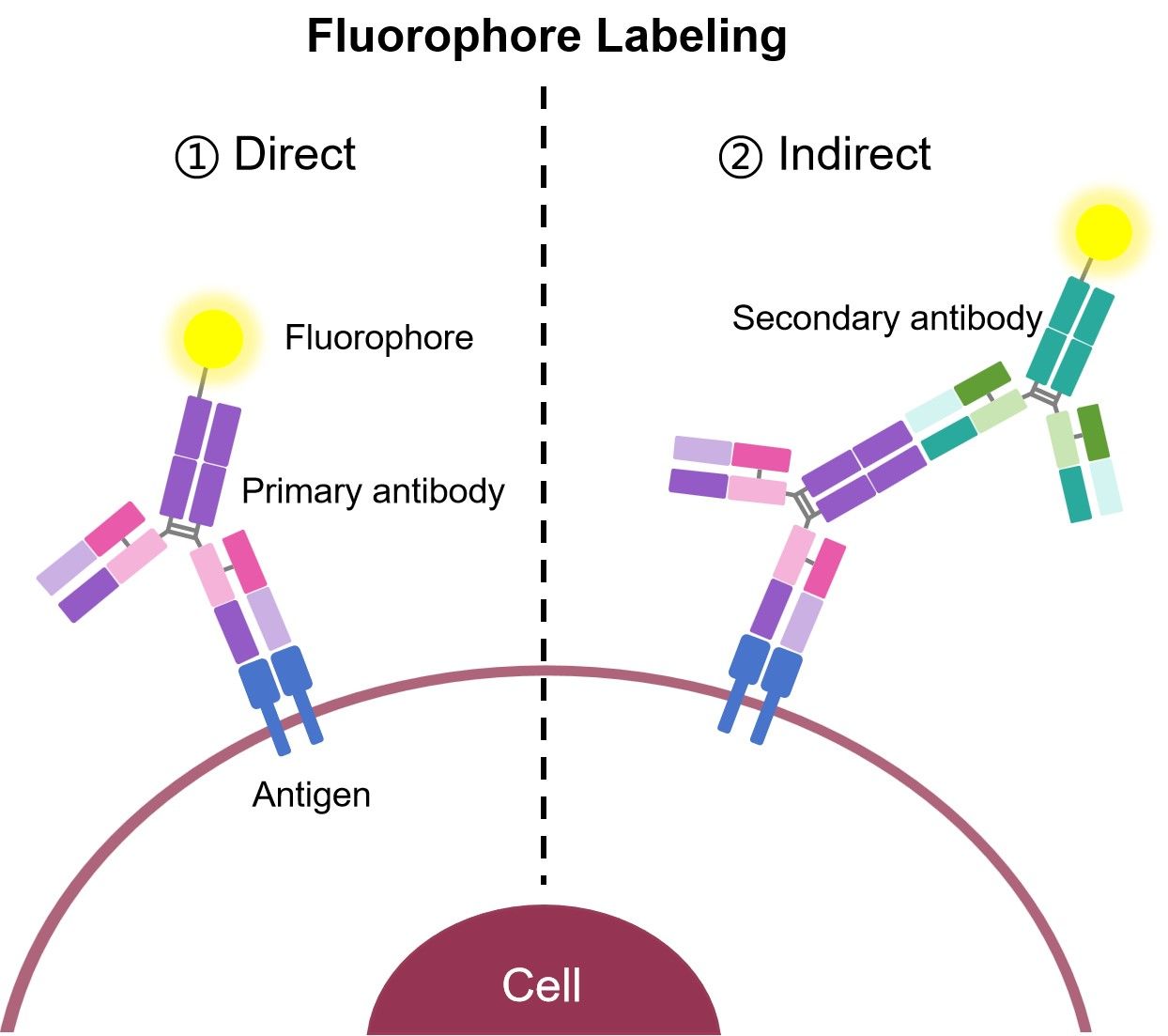 Fig.2 Two major IF types.
Fig.2 Two major IF types.
Direct Immunofluorescence (DIF)
DIF uses fluorescent-tagged antibodies to bind directly to the target antigen. DIF is a one-step procedure used to detect and localize immunoreactants deposited in vivo in the patient’s skin or mucosa. DIF must be considered when diagnosing oral mucosal conditions characterized by multifocal or diffuse blisters, chronic, ulcers or combination of these patterns. DIF can also be used to detect non-antibody targets in the skin, such as infectious organisms. In this case, a fluorophore-labeled primary antibody directed against the suspected antigen is used to detect the presence or absence of the organism. This technique is rapid and quite specific, but, owing to the limited number of antibodies that can bind to the specific target, it may be less sensitive than other microbiologic techniques.
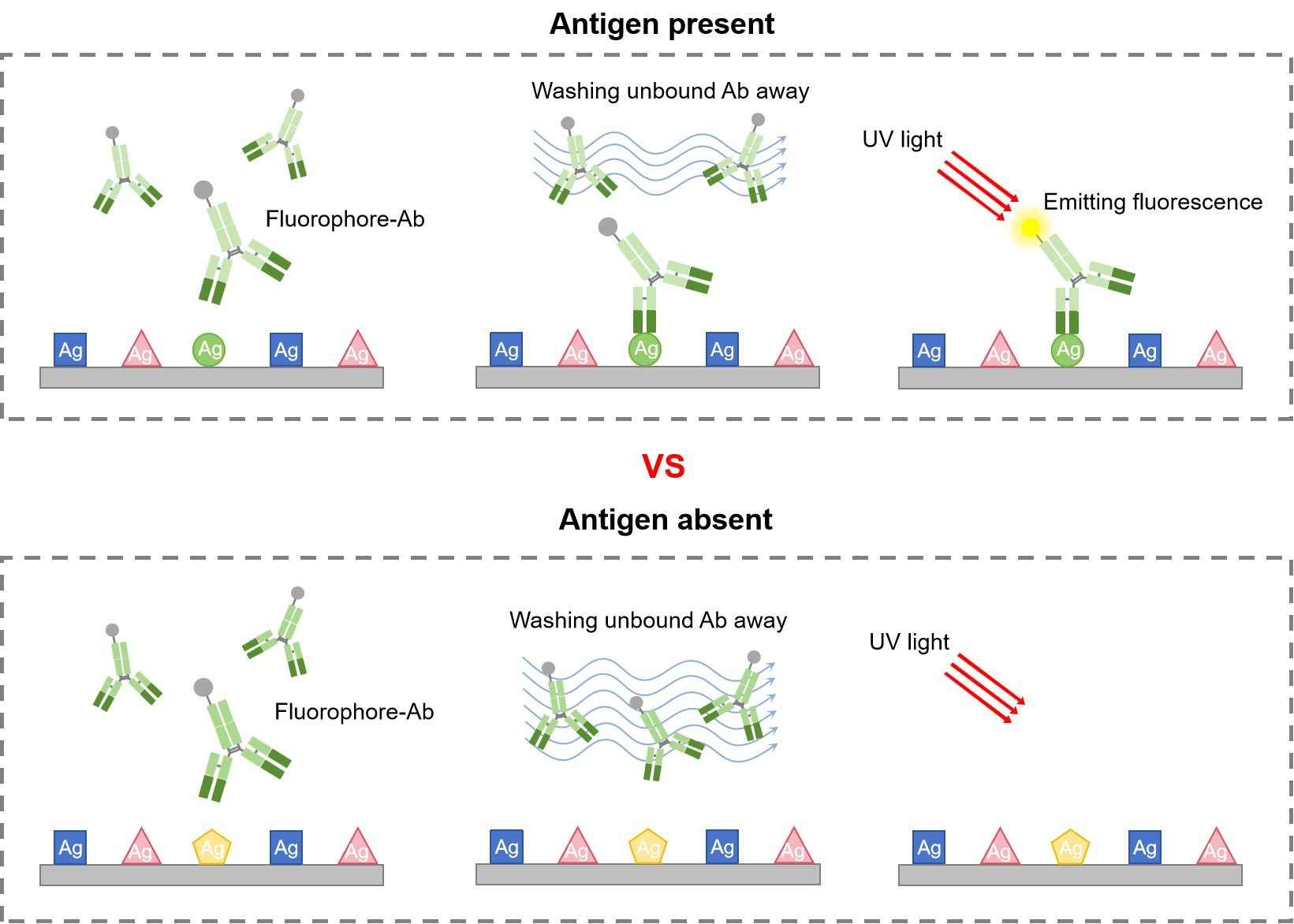 Fig.3 DIF procedure.
Fig.3 DIF procedure.
Complement Binding Technique
Complement binding IIF technique is a very sensitive, three-step serologic technique. Through this method, very small quantities of circulating antibodies are detected by means of their high affinity to fix complement. The complement can be demonstrated using FITC labeled anti-complement. The technique is more sensitive than routine IIF due to the amplification achieved through binding of more than one molecule of complement to each immunoglobulin (Ig) and the subsequent visualization of the multiple molecules of complement.
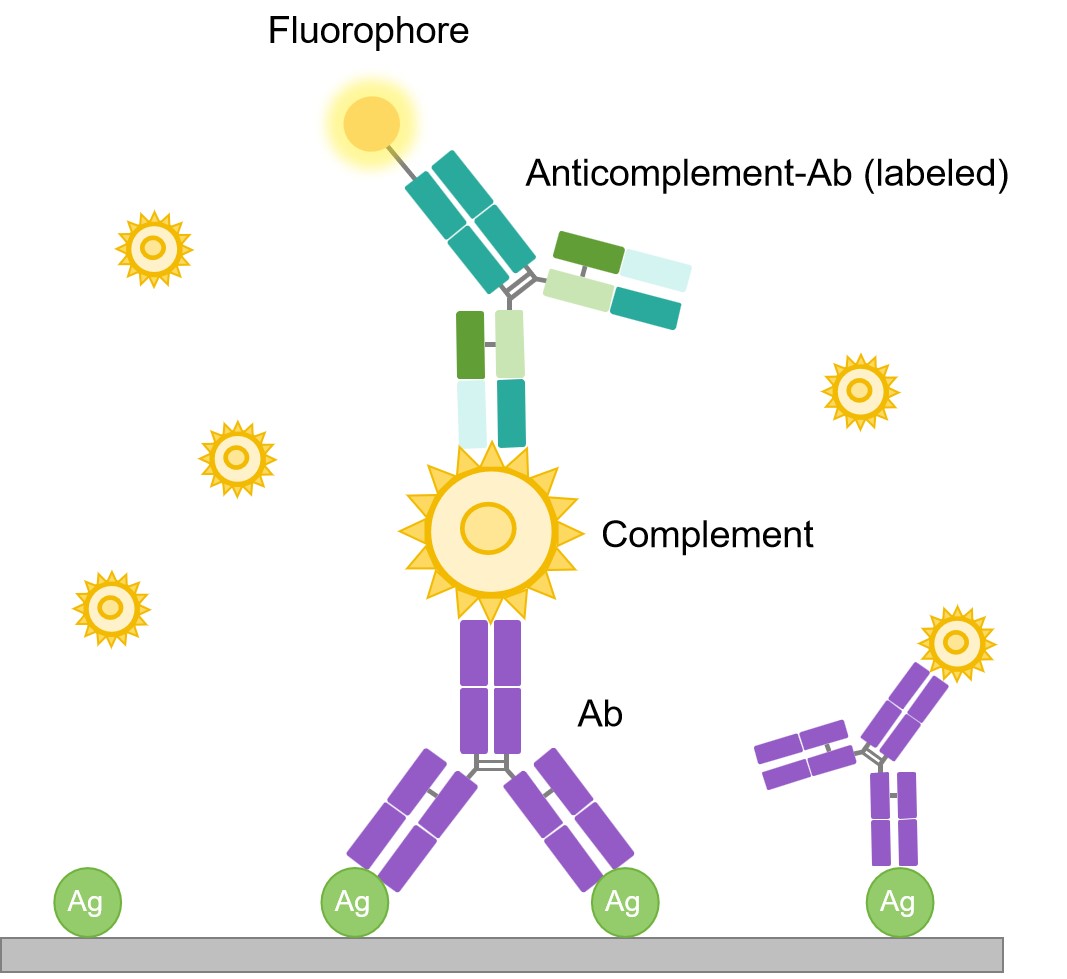 Fig.4 Complement fixation technique.
Fig.4 Complement fixation technique.
Variants of IF Technique
- Salt-Split Technique
- Antigenic Mapping Method
- Double Staining Method
- Sandwich Technique
- Calcium Enhancement Indirect Technique
Advantages of IF Technique
- Simple and reproducible
- Short procedure time of 1-3 h
- High sensitivity
- Substantial prognostic value, particularly for pemphigus cases
Limitations of Immunofluorescence Techniques
- Photobleaching
- Autofluorescence
- Fluorescence overlap
- Nonspecific fluorescence
- Sometimes multimerize or affect the kinetics and expression levels of target molecules
Applications
- Autoimmune diseases research
- Demonstration of enzymes, hormones, plasma proteins, cells
- Define antigen-antibody interactions at the subcellular level
- Identify viral, protozoal, bacterial and parasitic antigens
- Identify small cell surface structures, such as receptors on lymphocytes
Related Sections
Creative Biolabs presents a presentation that will be invaluable to beginners in IF and act as a fact-packed synopsis for those of you interested in teaching others about the virtues of this powerful application. To further assist learning, we have introduced the following sections for your viewing. Please refer to the corresponding section for details.
- Protocol of Immunofluorescence
- Troubleshooting of Immunofluorescence
- Multicolor Immunofluorescence (IF)
- Protocol of Multicolor Immunofluorescence (IF)
Reference
-
Dunst, Sebastian, and Pavel Tomancak. "Imaging flies by fluorescence microscopy: principles, technologies, and applications." Genetics 211.1 (2019): 15-34.
Distributed under Open Access license CC BY 4.0, without modification.
