Multicolor Immunofluorescence (IF)
Multicolor immunofluorescence (IF) staining analysis is a desirable tool in cell biology for most researchers. It allows researchers to study spatial and temporal colocalization of different targets. Multicolor IF can be performed either simultaneously using an antibody cocktail or sequentially by probing one antigen after another. Simultaneous and sequential staining follow the same general protocol, but considerable variation exists in the blocking and antibody incubation steps. Please refer to Protocol of Multicolor Immunofluorescence (IF) for details. In all multicolor staining protocols, the choice of primary and secondary antibodies is extremely important.
If you want to perform multicolor indirect immunofluorescence (IIF), the different primary antibodies must derive from different species in order to distinguish the immune complexes by labeling with fluorescence-coupled secondary antibodies. For instance, you are performing IIF with antibody against target A derived from rabbit, antibody against target B from rat and antibody against target C from mouse. When choosing the secondary antibodies, each of them specifically recognizes only one primary antibody. Besides, the fluorochromes of the three secondary antibodies must differ in their wavelength spectrum to discriminate their fluorescence signal in microscopic analysis. If performing a multicolor IIF for the first time, it is recommended to additionally stain the target structures in separate preparations and to compare these images with the multicolor image. Finally, you should take a shrewd look at your acquired data and compare it with your expectations and existing data on the same primary antibody.
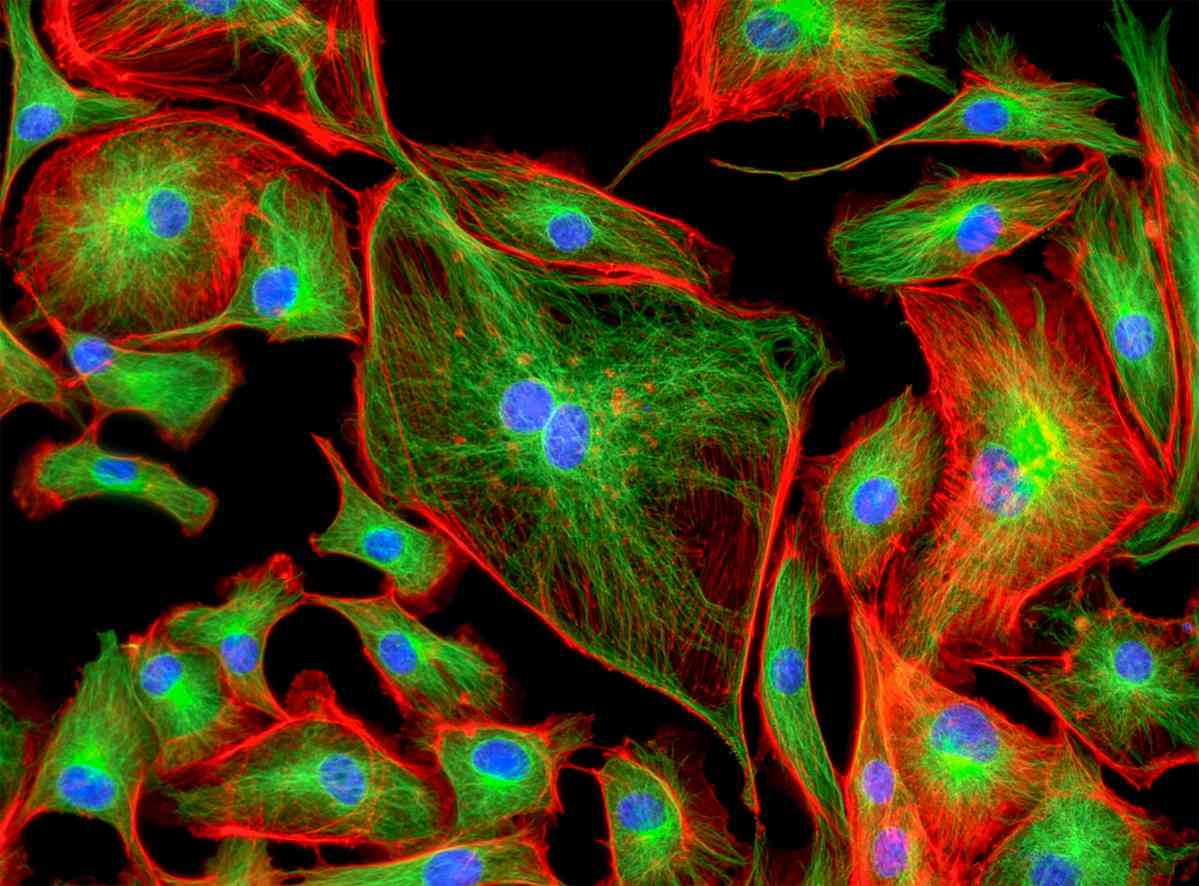
Our Tips for Better Multicolor IF Staining:
- A negative control lacking primary antibody should always be included to check the specificity of staining.
- For reducing fixative-induced auto-fluorescence, try quenching the fixation agent to eliminate any free aldehyde groups.
- Never let cells dry out and avoid dropping solutions directly on them. This will help maintain the proper cellular structural morphology and localization of proteins.
- When options are not limited, multicolor IF staining is best carried out by sequentially incubating cells with unlabeled-primary and labeled-secondary antibodies.
- When picking fluorochrome colors for labeled primary or secondary antibodies, one must consider their fluorescent emission spectrum. It is important for you to ensure that there is a minimal to negligible emission-spectra overlap between the selected fluorochromes.
- It is suggested to use the dimmest fluorochrome from one's available options to label the most abundant protein. Conversely, try detecting the least abundant protein with the fluorochrome having the highest quantum yield.
