LIFR Antibodies
Background
LIFR as a member of the type I cytokine receptor family, mediates cell fate determination and microenvironment adaptation through the JAK-STAT signaling pathway. The receptor regulates neural crest cell migration and organ morphogenesis during embryonic development, and participates in tissue homeostasis maintenance and damage repair in adulthood. In the evolution of malignant tumors, LIFR shows a dynamic expression pattern, and the abnormal activation of its signaling pathway is significantly correlated with the metastatic potential of breast cancer and the chemotherapy resistance of pancreatic cancer. It has become an important biomarker for tumor molecular classification and therapeutic target screening.
Structure of LIFR
As an important member of the type I cytokine receptor family, LIFR (leukemia inhibitory factor receptor) is cleverly assembled into a unique transmembrane glycoprotein structure by 1073 amino acids. Its three-dimensional conformation is like a sophisticated molecular machine: the extracellular ligand recognition region (ECD) accurately captures LIF/OSM signaling molecules through the β-clover conformation (CRH2 domain, amino acids 231-320); the transmembrane helical region (hydrophobic amino acids 792-816) forms a stable α-helical channel; the intracellular signal transduction region establishes a molecular docking platform with JAK kinase through the Box1/Box2 motif (amino acids 852-900).
At the microscopic structural level, the six pairs of cysteines in the extracellular region (Cys254-Cys265, etc.) are woven into three groups of disulfide bond networks, which stabilize the β-sandwich configuration like molecular rivets. The rigid α-helix formed by the PPXP repeat sequence (Pro853-Pro859) in the intracellular region produces hydrophobic interactions with the FERM domain of JAK1. This "lock-key matching" mechanism ensures the precise start and stop of signal transduction. When forming a heterodimer with the gp130 receptor, the β-chain 4-5 loop (amino acids 288-294) of the CRH2 domain produces charge complementarity with the IgD domain of gp130, and cooperates with the phosphorylation modification of the Ser974 site in the proximal membrane region to jointly regulate the cascade reaction of STAT3 tyrosine phosphorylation.
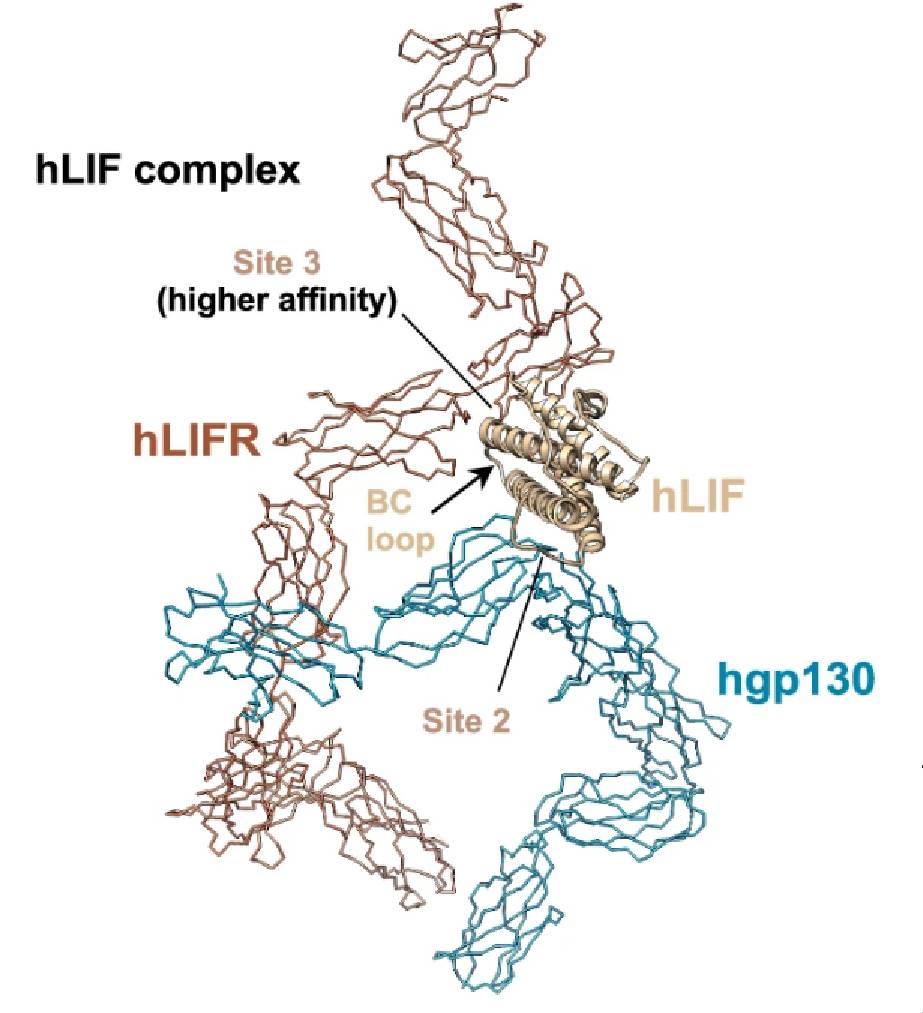 Fig. 1 Structural comparison of hLIF complex.1
Fig. 1 Structural comparison of hLIF complex.1
Functions of LIFR
LIFR (leukemia inhibitory factor receptor), as a member of the type I cytokine receptor family, mediates multidimensional biological functions through the JAK-STAT3 signaling pathway. During embryonic development, the receptor regulates the directional migration of neural crest cells and organ morphogenesis. Its ligand binding domain (CRH2, amino acids 231-320) specifically recognizes LIF/OSM ligands, triggering JAK kinase activation mediated by the intracellular Box1 motif (amino acids 852-862). In adult tissues, LIFR maintains hematopoietic stem cell homeostasis and coordinates skeletal muscle regeneration. Its function depends on the dynamic balance between microenvironmental signal input and STAT3 phosphorylation cascade reaction.
Under pathological conditions, LIFR exhibits functional heterogeneity: in breast cancer models, it enhances tumor metastasis potential by upregulating EMT-related transcription factors such as SNAI1 and TWIST1; while in the pancreatic cancer microenvironment, it promotes the formation of chemotherapy-resistant phenotypes by stabilizing the HIF-1α protein complex. This duality is related to its receptor dimerization mode - the heterodimer formed with gp130 can differentially regulate downstream signal output after phosphorylation modification at the Ser974 site.
Substantial progress has been made in targeted intervention strategies: a monoclonal antibody targeting the CRH2 domain (Clone# LFR-22B) reduced lung metastases by 63% in a triple-negative breast cancer mouse model; and a small molecule inhibitor targeting the Box1 motif (LFRi-109) increased the sensitivity of pancreatic cancer cells to gemcitabine by 4.2 times by blocking JAK1 recruitment. These findings provide a molecular basis for precision therapy based on the LIFR signaling pathway.
Applications of LIFR and LIFR Antibody in Literature
1. Zhou, Jieqing et al. "The ILEI/LIFR complex induces EMT via the Akt and ERK pathways in renal interstitial fibrosis." Journal of translational medicine 20.1 (2022):54. https://doi.org/10.1186/s12967-022-03265-2
This study demonstrates that activation of leukemia inhibitory factor receptor (LIFR) by interleukin-like EMT inducer (ILEI) drives tubular epithelial-mesenchymal transition (EMT) via Akt/ERK phosphorylation as a key mechanism of chronic kidney disease (CKD)-associated renal interstitial fibrosis (RIF) in children.
2. Edwards, C. M., et al. PTHrP intracrine actions divergently influence breast cancer growth through p27 and LIFR. Breast cancer research : BCR 26.1. (2024):34. https://doi.org/10.1186/s13058-024-01791-z
This study demonstrates that the C-terminal domain of parathyroid hormone-related protein (PTHrP) functions as an oncogenic switch in breast cancer progression by inhibiting leukemia inhibitory factor receptor (LIFR)-mediated p27 tumor suppression, whereas the nuclear localization signal (NLS) domain exerts a paradoxical growth regulatory role through differential regulation of the p27 signaling pathway.
3. Feng, Y., et al. OSMR deficiency aggravates pressure overload-induced cardiac hypertrophy by modulating macrophages and OSM/LIFR/STAT3 signalling. Journal of translational medicine 21.1 (2023):290. https://doi.org/10.1186/s12967-023-04163-x
This study demonstrates that leukemia inhibitory factor receptor (LIFR)-mediated STAT3 signaling exacerbates pressure overload-induced cardiac hypertrophy upon oncostatin M receptor (OSMR) deficiency, and identifies LIFR knockdown via Ad-shLIFR as a therapeutic strategy to mitigate pathological heart remodeling and STAT3 hyperactivation.
4. Mikelonis, Dawn et al. "Stüve-Wiedemann syndrome: LIFR and associated cytokines in clinical course and etiology." Orphanet journal of rare diseases 9 .34 (2014). https://doi.org/10.1186/1750-1172-9-34
This study establishes that autosomal recessive LIFR mutations drive Stüve-Wiedemann syndrome through destabilized mRNA translation and impaired JAK/STAT3 signaling transduction, while highlighting the urgent need for therapeutic strategies targeting this cytokine signaling pathway given the current absence of disease-modifying treatments.
5. Wang, Qun et al. "lncRNA LIFR-AS1 suppresses invasion and metastasis of non-small cell lung cancer via the miR-942-5p/ZNF471 axis." Cancer cell international 20.180. https://doi.org/10.1186/s12935-020-01228-5
This study elucidates that long non-coding RNA LIFR-AS1 suppresses non-small cell lung cancer metastasis through competitively sequestering oncogenic miR-942-5p to restore tumor suppressor ZNF471 expression, and identifies LIFR-AS1-based therapeutic strategies as promising interventions for restraining NSCLC progression given its significant correlation with clinical metastasis patterns and patient survival outcomes.
Creative Biolabs: LIFR Antibodies for Research
Creative Biolabs focuses on the research and development and production of LIFR (leukemia inhibitory factor receptor) related biological agents, providing a full range of solutions covering monoclonal antibodies and antibody arrays, suitable for various scientific research and industrial application scenarios. Core service modules include:
- Customized development: design specific LIFR binders according to customer needs
- Scaled production: establish a standardized GMP-level batch preparation system
- Technical guarantee: equipped with a professional team to provide production process optimization support
- Packaging management: implement strict quality control and standardized packaging process
For more details on our LIFR antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Zhou, Yi et al. "Structures of complete extracellular assemblies of type I and type II Oncostatin M receptor complexes." Nature communications 15.1 (2024):9776. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1038/s41467-024-54124-1
Anti-LIFR antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-DHFR Recombinant Antibody (D0821) (CBMAB-D0821-YC)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Mouse Anti-DLG1 Monolconal Antibody (4F3) (CBMAB-0225-CN)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Rabbit Anti-CCN1 Recombinant Antibody (CBWJC-3580) (CBMAB-C4816WJ)

-
Mouse Anti-FOSB Recombinant Antibody (CBXF-3593) (CBMAB-F2522-CQ)

-
Rabbit Anti-Acetyl-Histone H3 (Lys36) Recombinant Antibody (V2-623395) (CBMAB-CP0994-LY)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Mouse Anti-CD63 Recombinant Antibody (CBXC-1200) (CBMAB-C1467-CQ)

-
Mouse Anti-ACKR3 Recombinant Antibody (V2-261265) (CBMAB-C1023-LY)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7697) (CBMAB-1869CQ)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








