Western Blot (WB)
Overview
Western blot (WB) is a core technique in cell and molecular biology, which is used to detect the presence of a specific protein in a complex mixture extracted from cells. The WB procedure relies upon three key elements to accomplish this task: the separation of protein mixtures by size using gel electrophoresis, the efficient transfer of separated proteins to a solid support, and the specific detection of a target protein by appropriately matched antibodies. Once detected, the target protein will be visualized as a band on a blotting membrane, X-ray film, or an imaging system. Our WB protocol involves the following 5 main steps: sample preparation, SDS-PAGE gel electrophoresis, protein transfer, immunoblotting, detection. Creative Biolabs provides very detailed steps instruction for each one. Please refer to Western Blot Protocols & Troubleshooting & Guide for more information.
Detection Method
Detailed procedures for detection of a WB may vary widely. One common variation involves direct vs. indirect detection. The following diagram and tables show the specific differences between them.
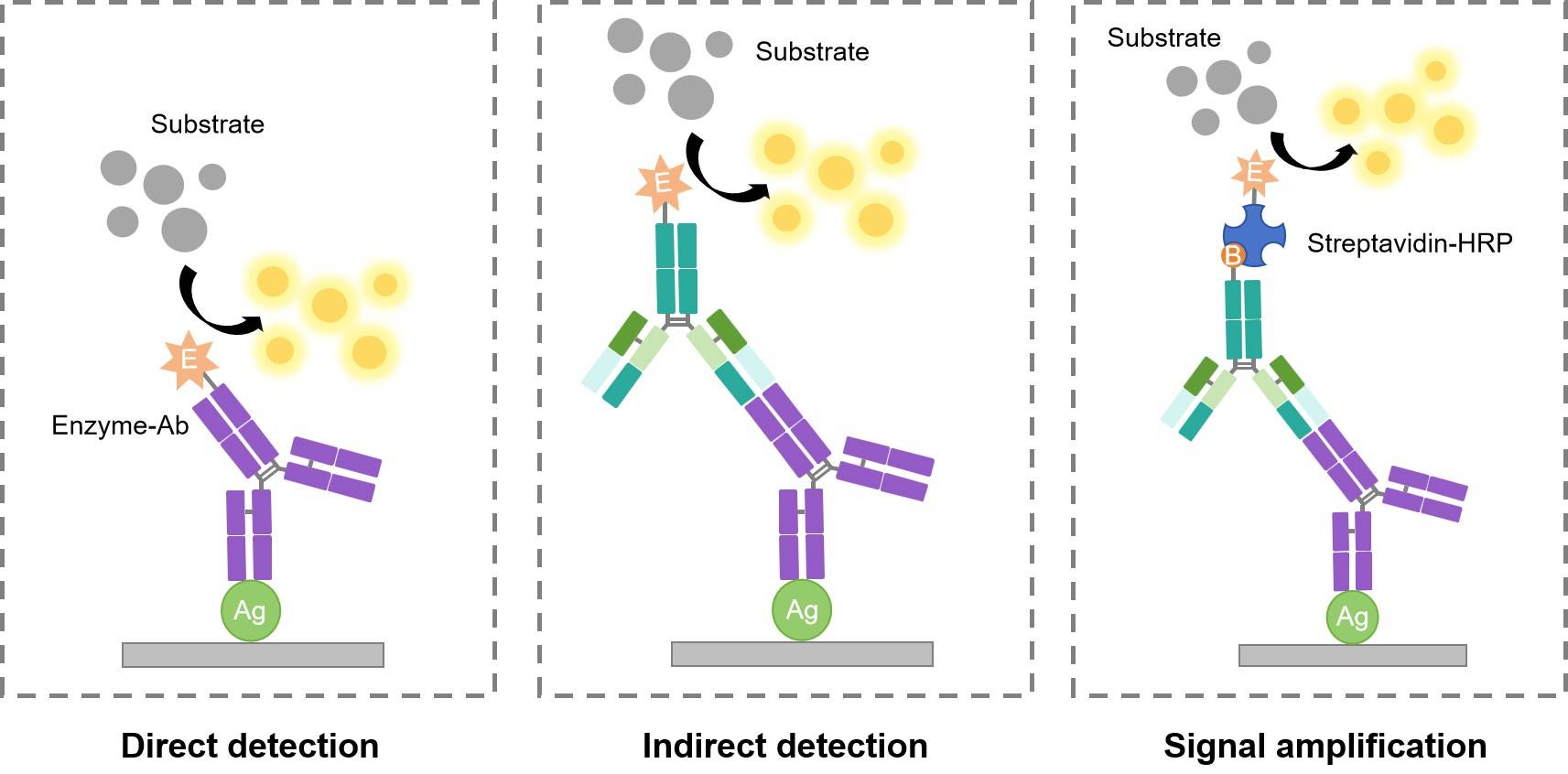
Table 1. Direct Detection Method
| Advantages | Disadvantages |
|
|
|
|
|
|
|
Table 2. Indirect Detection Method
| Advantages | Disadvantages |
|
|
|
|
|
|
|
|
|
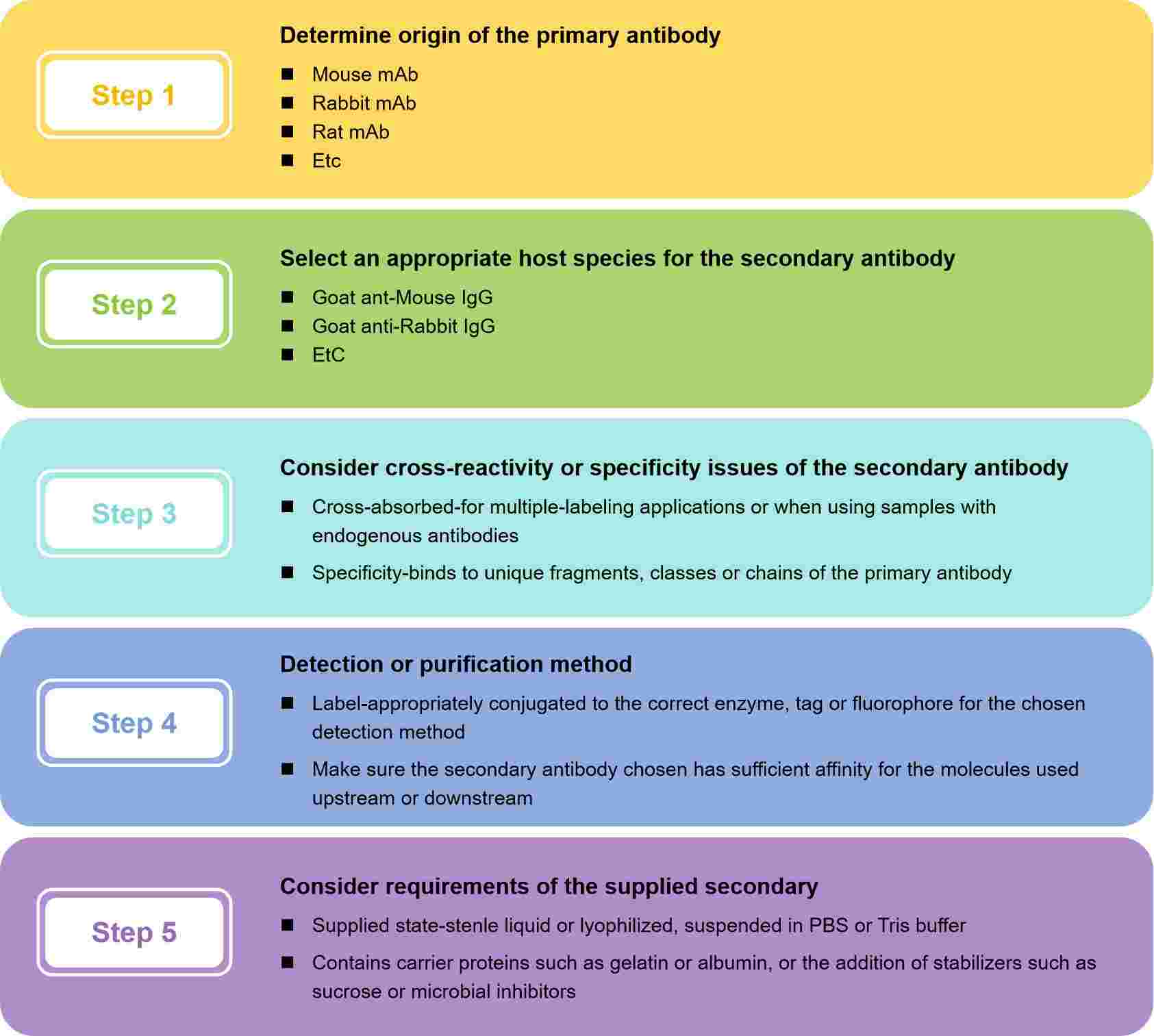
How to Choose Appropriate Secondary Antibody?
Protein Electrophoresis
Protein electrophoresis is a standard laboratory technique by which charged protein molecules are transported through a solvent by an electrical field. Most biological molecules carry a net charge at any pH other than their isoelectric point and will migrate at a rate proportional to their charge density. Denaturing and reducing SDS-PAGE with a discontinuous buffer system is the most widely used electrophoresis technique and separates proteins primarily by mass. Non-denaturing PAGE (also known as native-PAGE) separates proteins according to their mass/charge ratio. Two-dimensional (2D) PAGE separates proteins by native isoelectric point in the first dimension and by mass in the second dimension.
SDS-PAGE vs. Native-PAGE
- In SDS-PAGE, the gel is in a buffer containing SDS which denatures proteins by wrapping around the polypeptide backbone. By heating the protein sample between 90-100°C in the presence of excess SDS and thiol reagent, disulfide bonds are cleaved, and the protein is fully dissociated into its subunits. The intrinsic charges of the polypeptide are insignificant compared to the negative charges provided by the bound detergent so that the SDS-polypeptide complexes have essentially the same negative charge and shape. Consequently, proteins migrate through the gel strictly according to polypeptide size with very little effect from compositional differences.
- In native-PAGE, proteins are separated according to the net charge, size, and shape of their native structure. Because no denaturants are used in native-PAGE, subunit interactions within a multimeric protein are generally retained and information can be gained about the quaternary structure. Following electrophoresis, proteins can be recovered from a native gel by passive diffusion or electro-elution.
Recent Progresses
Although WB is a relatively mature technology, there are still researchers working on it to upgrade this technique. The development of improved detection methods can expand multiplexing capabilities and push detection limits. Progress in these areas will likely continue at a rapid pace as improved method for microfluidics, microscale analysis systems, and label-free biosensing continue to advance the field.
- Multiplexed Microfluidic Probing Following WB
- Microarray Spotting of Samples for WB
- Capillary Gel Electrophoresis Coupled with Direct Blotting
- Sieving Microchip Electrophoresis Coupled with Direct Blotting
- Fully Integrated Microchip for WB
- On-Capillary Protein Immobilization Approaches
- In-Gel Protein Immobilization Approaches
More WB Resource
Western Blot Protocols & Troubleshooting & Guide
Sample Preparation in Western Blot Assay
Protein Transfer from Gel to Membrane in Western Blot Assay
Running an SDS-PAGE Gel in Western Blot Assay
Western Blot Illustrated Assay
