Antibody Production
Monoclonal antibodies (mAbs) are widely used in biomedical research and medicine to fight, diagnose and research diseases and to develop and test new drugs. However, the mouse ascites method of mAb production (such as Hybridoma) is significantly limited due to the substantial pain and distress involved, and raised serious animal welfare concerns. Fortunately, an alternative to animal-based mAbs appears, namely recombinant antibodies (rAb). rAb is produced using antibody genes (screening by phage display) in a laboratory or taken from human cells, with completely no need for animals. Currently, rAb can meet all applications in which traditional mAbs are used and present inherent advantages over their traditional animal-derived counterparts.
Creative Biolabs has a world-class technical team to provide the most comprehensive list of rAb products and customized rAb development services for clients. We believe our off-the-shelf products and high-quality services will support your needs.
Antibody Production Methods: Hybridoma vs Phage Display
- Hybridoma
Hybridoma was first developed in 1975 by George Köhler and César Milstein. This technology is widely used for the antibody production based on the fusion of mouse immunized B spleen cells with myeloma cells. Antibody of interest is produced by the immortal B cells, and then the best clones are screened to obtain monoclonal antibodies with the desired antigen affinity.
Hybridoma development represents one of the most traditional methods for the generation of monoclonal antibodies, which can produce highly sensitive binders and make them particularly adapted for assay development. Importantly, the integration of the mammalian origin of the cells and in vivo post-translational modifications significantly decrease the risk of aggregation or recognition failures.
Hybridomas technology display unparalleled advantages that make it also used for therapeutic antibody development. However, it is worth mentioning that hybridomas also have some significant drawbacks. Firstly, the antibody development using hybridoma is very time-consuming, which will take on average between 6 and 8 months to obtain a reasonable amount of monoclonal antibodies (against a few weeks for phage display). Secondly, the mouse origin of antibodies means further humanization for therapeutic purposes, which significantly leads to additional costs. Therefore, hybridoma development is being progressively replaced by faster, more appropriate techniques for biotherapeutics development such as recombinant antibody production using phage display technology.
- Phage Display
Phage display method was created by Smith in 1985. Simply, the process refers to a gene sequence coding for a particular antibody that is integrated into the DNA sequence of a filamentous bacteriophage, which can be expressed on the surface of the bacteriophage capsid. The phage infects Escherichia coli and then continues to display new phage in the host cell. After the display process, a large number of antibodies are produced rapidly. Thus, a library of naïve or immune phage is constituted and can be used to detect an antigen-antibody interaction of interest through screening methods. Libraries can be generated from any animal to directly screen antibodies. Subsequently, the sequence is obtained easily that facilitates further engineering and recombinant antibody production.
Unlike hybridoma development, when a naive library is available, the process can be very fast and usually only needs a few weeks to screen a greater diversity of antibodies. However, phage display is more expensive compared with hybridoma, and it is possible to result in a lower affinity when panning naïve libraries.
| Hybridoma | Phage Display | |
| Advantages |
|
|
| Disadvantages |
|
|
Development of rAbs
rAbs are a type of antibody fragments produced using recombinant antibody coding genes that are obtained from library display. Most of recombinant antibodies are composed of a heavy and light chain of the variable region of immunoglobulin. rAbs have different forms such as the single chain variable fragment (scFv), Fab fragments and bispecific recombinant antibodies, etc. rAbs are displaying promising traits exploitable in human medicine and research. Recombinant antibodies possess a number of advantages that make them a popular subject of exploration and new production against specific targets.
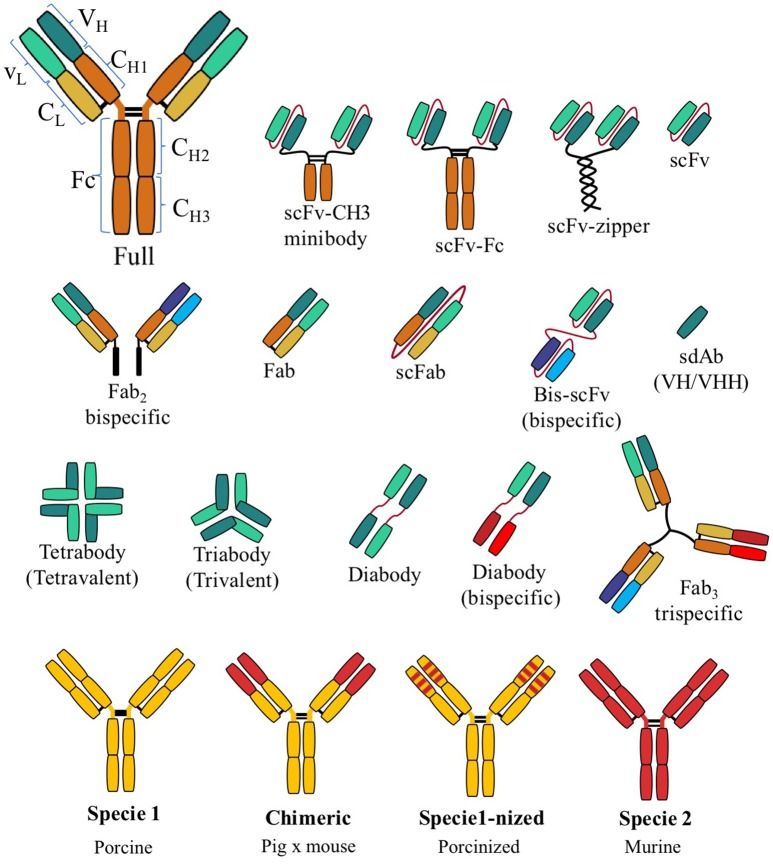 Fig.1 Recombinant antibody fragments.1
Fig.1 Recombinant antibody fragments.1
The emergence of recombinant DNA technology endows the promise for recombinant antibody production. Generally, the development of recombinant antibodies includes five steps:
- Antibody Gene Libraries: Antibodies are composed of heavy and light chains that each has a variable and a constant region. The variable regions contribute to antigen binding. The constant region determines the class, or isotype of an antibody. An antibody gene library is a collection of microorganisms with the genes of the variable regions of different antibodies. The variable region genes can either be synthesized in vitro or obtain from the antibody-producing B cells. Each variable region gene is spliced into a vector that is inserted into a microorganism.
- Library Display: Antibody library vectors produce a protein on the surface of viral particles or cellular membranes via genetic instructions. Variable region genes are spliced into these instructions so that a fusion protein is created consisting of a fully functional antibody fragment and the surface protein when the vector is inserted in a microorganism. In yeast display platforms, variable region genes are often fused to the yeast Aga2p gene. In phage display platforms, variable region genes are fused to the pIII phage coat gene frequently. The antibody library vectors are inserted into bacteria, subsequently, the bacteria are infected with modified phage.
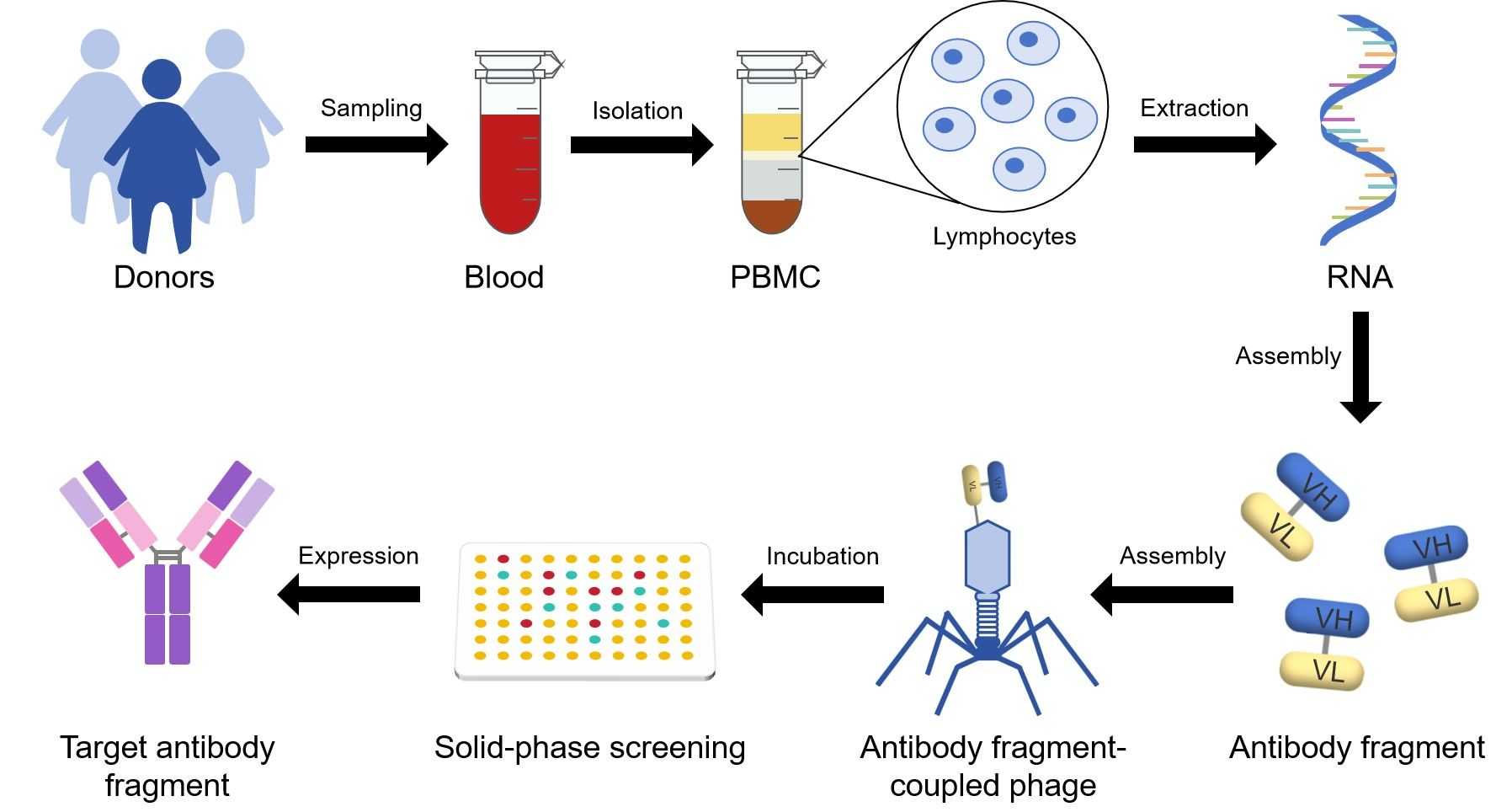 Fig.2 General scheme for the preparation and selection of a naive recombinant antibody library.
Fig.2 General scheme for the preparation and selection of a naive recombinant antibody library.
- Antibody Isolation: Upon the rAbs are displayed, paramagnetic beads, fluorescence-activated cell sorting (FACS), and/or Enzyme-Linked Immunosorbent Assays (ELISAs) can be used to isolate individual antibodies that can recognize a specific antigen target.
- Modification: Promising antibodies are produced in greater quantities and put through the selection process again to enrich for the highest performing candidates. If the affinities of the candidates are not strong enough, the antibodies can be modified to improve the affinities by random or rational mutagenesis methods, such as the affinity maturation.
- Antibody Expression: Once a satisfied antibody is chosen, the genes of the antibody are transferred via expression vectors into an expression system for the expression of foreign proteins, such as the bacteria, yeast, or mammalian cell lines specially designed. The option of vector and expression system depends on the type of antibody. In general, full-length antibody often is expressed in yeast and mammalian expression systems. Fragment antibodies frequently to be produced with bacterial expression systems that are quick and inexpensive.
Advantages of Recombinant Antibody Use
- Non-animal technology: Recombinant antibodies completely derived from synthetic or human antibody libraries are an entirely non-animal technology.
- Speed: Once an antibody library is established, a recombinant antibody can be produced within 8 weeks. However, traditional hybridoma technology needs about 4 or more months.
- Control: Traditional mAb technology makes researchers lose control once injecting the antigen into an animal. In contrast, the recombinant antibody production process can be regulated by adjusting experimental conditions to favor the isolation of antibodies against specific antigens or antigen characteristics.
- Isotype conversion: An antibody fragment can be easily converted into any antibody isotype (such as IgA, IgM IgG) from any species by adding the appropriate constant domain.
- Applications: Recombinant antibodies can be used in all applications.
Recombinant Antibody Production at Creative Biolabs
Creative Biolabs is a leading provider of antibody products and custom recombinant antibody production services. We have various expression systems to meet the production for different types rAbs such as full-length antibodies, scFv, Fab/Fabʹ, sdAb/VHH, Fc fusion proteins, and chimeric antibody and so on. With deep understanding of the each procedure in antibody development, Creative Biolabs is fully prepared to provide customized antibody production service not only rAbs for our worldwide customers. For further information and a detailed quote please contact us, we will be happy to assist you.
Work flow of our rAb production includes:
- Gene Cloning
- Transformation of Expression Plasmids
- Small-scale Expression
- Large-scale Expression & Bacterial Disruption
- Purification of Fusion Proteins
- Protein Concentration & Concentration Determination
- Detection Assays
Reference
-
Bustamante-Córdova, Lorena, Edgar A. Melgoza-González, and Jesús Hernández. "Recombinant antibodies in veterinary medicine: An update." Frontiers in Veterinary Science 5 (2018): 175.
Distributed under Open Access license CC BY 4.0, without modification.
