Purification of Fusion Proteins
Creative Biolabs provides the production of various recombinant antibody including full-length IgG antibody, F(ab) and (Fab’)2 antibody fragments, and antibody fusion proteins. Our custom antibody production capabilities include construct design, antibody production and affinity purification. Regarding our purification services, we offer besides Tag recombinant protein purification, we also provide size exclusion chromatography of up to 10 grams of antibody in a single production run, buffer exchange by dialysis, endotoxin testing, analytical SEC, and SDS-PAGE.
Introduction
The production of a recombinant antibody requires a suitable expression system and a practicable purification strategy. Most of the smaller antibody formats such as single-chain Fv, Fab fragments or (single-chain) diabodies without glycosylation sites can be produced in the periplasm of Escherichia coli with yields of ~1 mg/L in standard batch cultures. However, some more complex antibody fusion proteins or molecules containing CH2 or Fc domains require expression in CHO or HEK293 cells. Besides, some scFv or diabody molecule produced in E. coli often present weak solubility that also needs a eukaryotic expression system. The choice of an optimal purification tag is another critical factor for protein purification in the laboratory scale. The relatively weak selectivity of the Ni-NTA matrix for the common 6×histidine tag endows one-step immobilized metal affinity chromatography (IMAC) purification with a desirable protein quality only from pure tissue culture supernatants. By contrast, the protein purity by IMAC is relatively crude, a time-consuming second purification step, e.g., ion exchange chromatography is essential for sensitive applications. In addition, the presence of a 6×histidine tag in recombinant proteins may influence protein activity, biophysical properties, or show immunogenicity. Thus, some alternative tags display more specific isolation of recombinant proteins.
Purification of Fusion Proteins
- Expression and Purification of 6×His-Tagged Proteins (E. coli)
The affinity tag 6×histidine commonly located at the C-terminus of the molecule can be used for purification and detection.
- Harvest the cells (6,000 ×g, 10 min, 4°C).
- Resuspend the pellet in 50 ml PPB per liter bacterial culture.
- Add 250 μl lysozyme solution (final concentration: 2.5 mg lysozyme/50 ml PPB), mix by inverting and incubate for 20 min on ice.
- Add 0.5 ml MgSO4 solution (final concentration: 10 mM) to stabilize the spheroplasts and mix by inverting.
- Centrifuge (10,000 ×g, 10 min, 4°C) and collect the periplasmic preparation (supernatant).
- Dialyze the periplasmic preparation against IMAC loading buffer at 4°C overnight using a magnetic stirrer.
- (Day 3) Collect periplasmic preparation and centrifuge (10,000 ×g, 10 min, 4°C) to pellet aggregates and residual cell debris.
- For equilibrating Ni-NTA beads, transfer an appropriate amount of Ni-NTA beads into a 15 ml tube and centrifuge (2,000 ×g, 5 min, 4°C). Discard the supernatant and resuspend the beads in IMAC loading buffer (tenfold of the Ni-NTA bed volume). Repeat this step once.
- Transfer the Ni-NTA beads to the periplasmic preparation and incubate rolling at 4°C for at least 2 h.
- Load the Ni-NTA bead suspension on a chromatography column and drain by gravity flow. Save the flow-through for analysis on SDS-PAGE.
- For washing, load 16 column bed volumes (CV) of IMAC washing buffer on the column. Let the IMAC washing buffer drain completely by gravity flow before adding fresh buffer. Repeat this step until the wash fractions do not contain any protein. Save the wash fractions separately for analysis on SDS-PAGE.
- For elution, load 1 CV IMAC elution buffer on the column. Again, let the IMAC elution buffer drain completely before adding fresh buffer. Repeat this step until all protein is eluted from the Ni-NTA beads. Check the protein content of the elution fractions by protein assay. Pool the fractions that contain sufficient amounts of protein to a main fraction, the other protein containing fractions can be pooled to a side fraction.
- Dialyze main and side fraction against PBS at 4 °C overnight using a magnetic stirrer (at least a dilution of 1:1,000).
- (Day 4) Centrifuge the dialyzed protein solution to pellet aggregates (8,000 ×g, 1 min, 4°C). Collect the clear supernatant and store the protein either in the fridge (stable for some weeks) or at -20°C (stable for several months).
- Perform a SDS-PAGE analysis to check the quality of the purification. Therefore, load 5 μl of main and side fraction and 20 μl of the flow-through and the wash fractions on the gel.
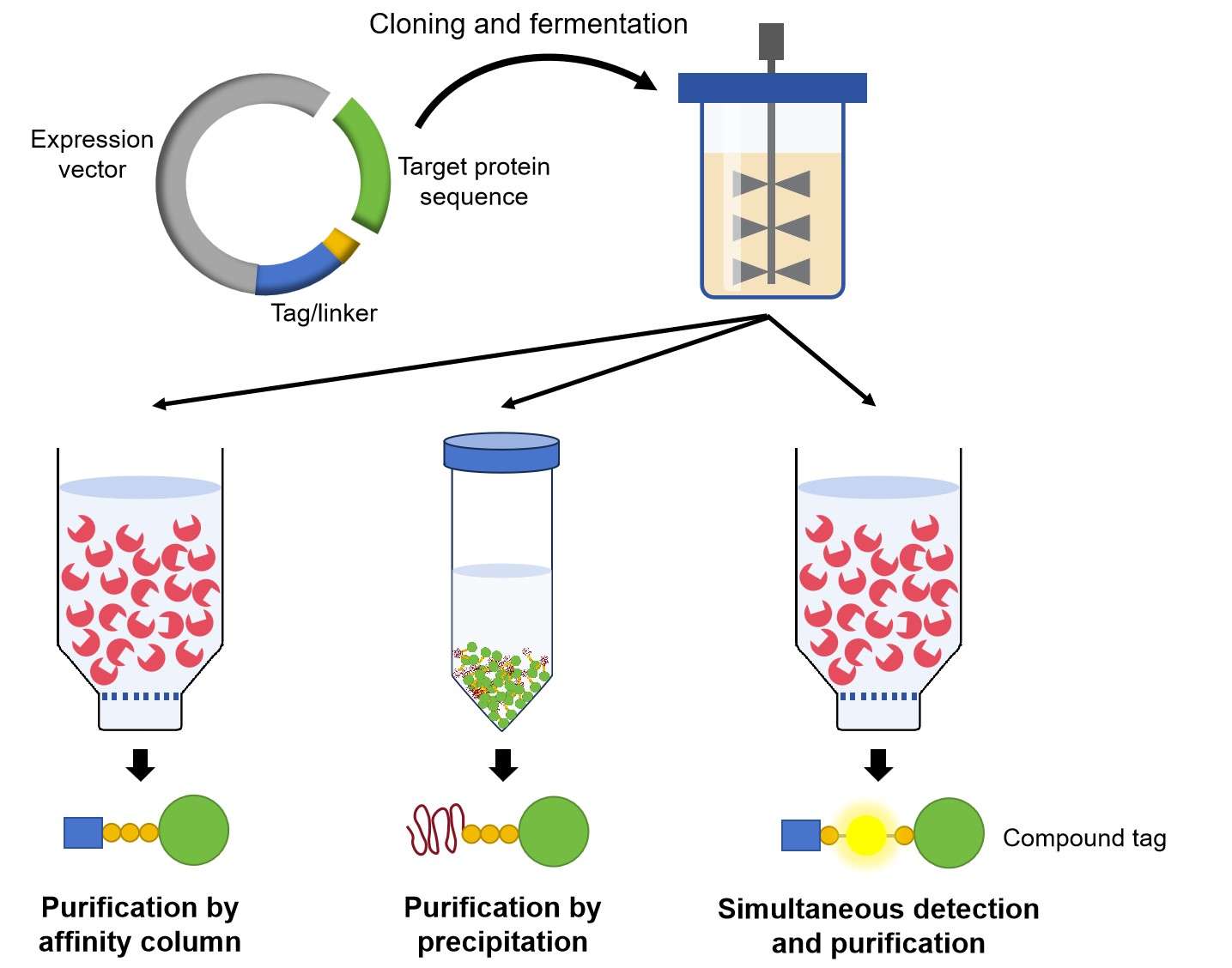 Fig.1 The purification of His-tagged proteins.
Fig.1 The purification of His-tagged proteins.
- Purification of DYKDDDDK-tagged Proteins Expressed in Mammalian Cells
This protocol depicts an efficient method to obtain high yields for purification of up to 10 mg of DYKDDDDK-tagged recombinant protein/ antibody from ~200 to ~900 ml tissue culture supernatant.
- Mount the empty, clean chromatography column on a stand. Rinse the column twice with TBS, close the outlet of the column, and leave 2 ml TBS in the column.
- Pipette an appropriate volume of M2 agarose in the column and allow the resin to settle for 5 min. Open the column outlet and allow the buffer to flow out.
- Use 5 ml of TBS to wash adherent M2 agarose from the pipette and transfer the TBS to the column.
- Connect the column lid with the silicone tubing and use the peristaltic pump to speed up draining by pressing air on the column. Drain the column.
- Load 5-10 ml TBS on the column without disturbing the gel bed and let it drain completely.
- For washing, apply three sequential column bed volumes (CV) of 0.1 M glycine HCl, pH 3.5 without disturbing the gel bed while loading. Let each aliquot drain completely before adding the next.
- Repeat step 5. Then, fill the column with TBS for a second time and let it drain until 1-2 ml TBS remains above the gel bed. For equilibration, pump 10 CV of TBS through the material. After that, leave ~1-2 ml of TBS above the gel bed and close the column outlet.
- Distribute the tissue culture supernatant to one or more 500 ml centrifuge bottles. Resuspend the M2 agarose in the overlaying TBS by pipetting up and down with a 5 ml serological pipette and distribute the suspension equally to the centrifuge bottles. Add TBS to completely recover the M2 agarose from the column. Fill the empty column with TBS.
- Incubate the centrifuge bottles on a roller mixer at 4°C for ~2 h.
- Centrifuge bottles at 1,000-1,500 ×g for 20 min.
- Carefully remove the supernatant from the centrifuge bottles without disturbing the resin pellet and keep it in a separate bottle. Leave a few milliliters of supernatant to facilitate resuspension of the resin. Place the centrifuge bottles containing the resin pellets on ice.
- To maximize the resin recovery from the batch incubation, load the supernatant on the empty column by pumping at a high flow rate (8-10 ml/min). Reduce the flow rate, if column backpressure rises (Optional).
- Use a 1 ml pipette to resuspend the resin and load the material on the empty column. Wash the 1 ml pipette and the wall of the centrifuge bottle with ~3 ml of TBS and load the wash on the column. Drain column completely to form a compact column bed, but do not let the material run dry.
- For washing, load five sequential CV of TBS and let each volume drain completely before adding the next.
- For elution, load six sequential CV of TBS with 100 μg/ml of DYKDDDDK peptide and let each volume drain completely before adding the next.
- Combine fractions containing protein and dialyze the eluate in at least 5 L of PBS at 4°C overnight.
- Repeat steps 6 and 7, but add 0.02 % sodium azide to the TBS. Store the M2 agarose column at 4°C.
- The next day, recover the dialyzed eluate and centrifuge at 5,000 ×g for 10 min at 4°C. Transfer supernatant into a fresh tube.
- Wash a centrifugal concentrator twice with PBS according to the manufacturer’s instruction and remove the remaining PBS from the concentrate chamber after the second wash. Fill the concentrate chamber with the eluate and spin the device at the recommended speed (3,000-4,000 ×g for 10,000 or 50,000 Da MW cutoff membranes) at 4°C until the eluate is concentrated to a volume of ~4 ml.
- Add ~15 ml PBS to the concentrate chamber and mix gently by pipetting. Spin the device until the eluate is concentrated to a volume of ~4 ml.
- Repeat step 20, but spin the device until the eluate is concentrated to a volume of ~1-3 ml.
- Mix the concentrated eluate in the retentate chamber by pipetting it up and down with a 200 μl pipette tip. Use a syringe with a needle to recover the concentrated protein.
- Filter the concentrate with the 13 mm syringe filter into a sterile tube. Vortex the sample shortly and measure the protein concentration. Aliquot the purified protein and store it frozen or at 4°C.
For more information for the purification of fusion proteins with other tags, please contact us.
What Can We Do For You?
Creative Biolabs has been working on recombinant protein expression and antibody development for many years. We provide customers with customized protein purification services, including tag/tag free recombinant proteins, purification and separation of natural proteins. Our well-established purification platform and technology enable simultaneous large-scale, scanning protein purification and separation to save your time and money. For more information on how to access this service, please contact us.
Work flow of rAb production includes:


