Small-scale Expression
Recombinant antibodies (rAbs) are highly specific detection probes used in research, diagnostics. Over the last two decades, rAbs have emerged as the fastest growing class of therapeutic proteins. Creative Biolabs has professional antibody expression platform with more than ten years of process optimization and production experience, providing one-stop services from gene synthesis to high-quality antibody production. We have successfully delivered thousands of recombinant antibodies manufacturing programs to customers worldwide and have established good cooperation relationships with leading pharmaceutical companies in the world. We also provide a small-scale expression service to meet your antibody production.
Small-scale Expression of Recombinant Antibodies
Recombinant antibodies are widely used in biological and biomedical research, and have become a commonly used tool. rAbs can be expressed in bacteria, in yeast, in insect cells or in mammalian cells. Of all the expression hosts, E.coli is usually the first choice for small-scale expression of rAbs due to its ease of manipulation and low cost. Creative Biolabs is equipped with experienced scientific team and well-developed recombinant protein expression systems to serve the biopharmacetutical and life science field fully customized recombinant protein production services. Besides, we also provide antibody production using other expression systems from the prokaryotic systems and eukaryotic systems with both structural integrity and functional activity.
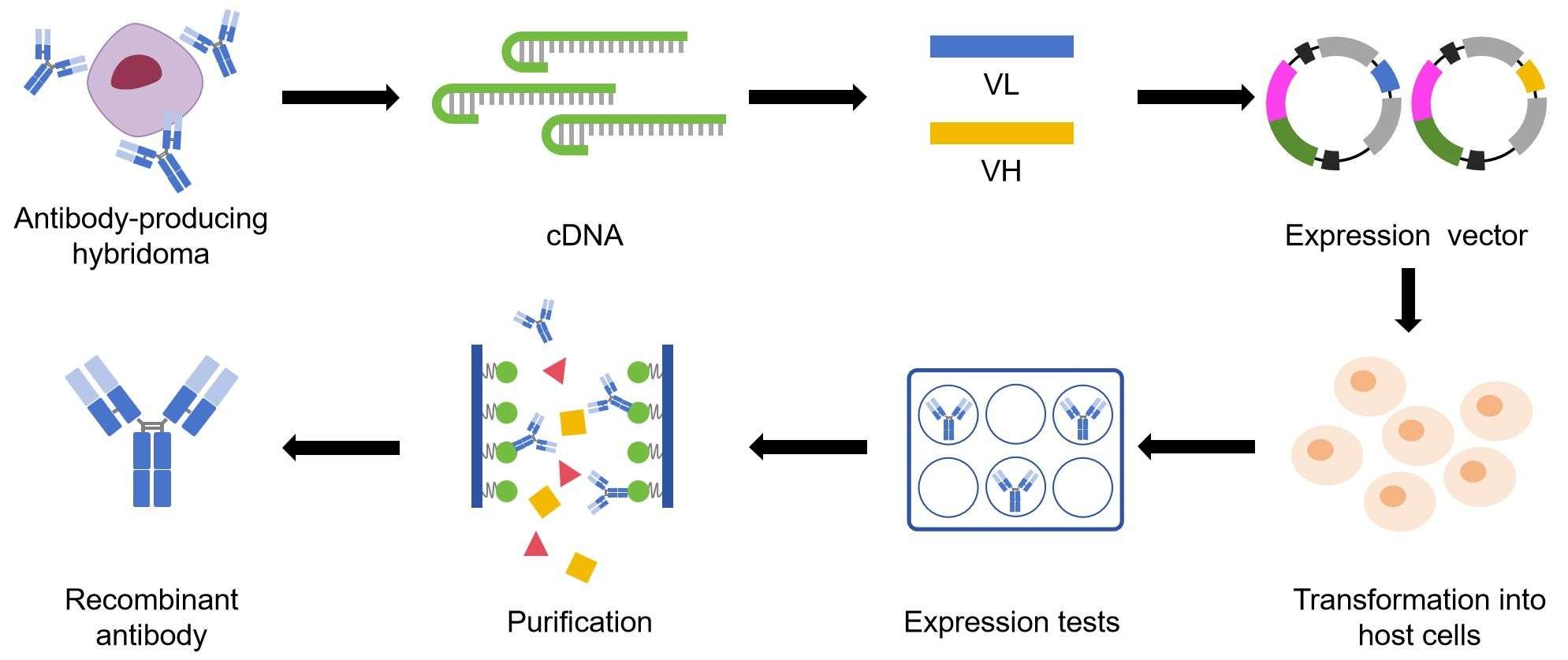 Fig.1 Small-scale expression steps for monoclonal antibody cloning from hybridoma.
Fig.1 Small-scale expression steps for monoclonal antibody cloning from hybridoma.
Steps of Small-scale Recombinant antibodies expression
-
Molecular cloning
The gene of antibody is inserted into expression vector. Usually the antibody protein is fused to a protein tag to facilitate expression and purification. The protein tag can be inserted to the N-terminus or C-terminus of the antibody gene for fusion protein expression. A protease cleavage site can be inserted between the antibody protein and the tag, which is used to remove the tag after purification. In addition, the expression vector also possesses antibiotic resistance markers that are used for clone selection.
-
Transformation of the expression vector into host cells
Competent cells have the ability to take up foreign DNA that contains the antibody protein for expression. Steps include:
- Add the target vector into thawed competent cells.
- Incubate the cells on ice, then treat with heat-shock and ice-shock.
- Add liquid medium into treated competent cells for recovery.
- Plate the transformed cells onto agar plates containing corresponding antibiotic for clone selection.
-
Expression tests
- Transform the chosen E.coli strain competent cells for expression with the constructed expression vector.
- Select the appropriate colony by antibiotic screening.
- Incubate the correct colony harboring the recombinant plasmid in LB until OD600 reached to a certain value.
- Divide the culture, equilibrate at different chosen temperatures, and induce expression by addition of isopropyl-β-D-thiogalactoside (IPTG).
- Continue cell growth and take samples at different time points after induction.
- The remaining cultures were allowed to continue growing and eventually harvested.
- All the samples were then lysed by sonication and then centrifuged to separate the supernatant and pellet for sodium-dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
- Subject the re-suspended cells to a single freeze-thaw cycle prior to DNase digestion.
- Centrifuge the lysed cell to remove the cell debris and filter the supernatant through a filter membrane.
-
Protein purification
- Cell lysis. Lysis process often uses mechanical lysis by sonication or lysis by freeze-thaw plus with lysozyme and lysis buffer. PH and salt concentration of the lysis buffer needs to be optimized to enhance protein solubility and stability according to the physiochemical property of the target protein. Protease inhibitors and reducing agent such as Tris (2-carboxyethyl) phosphine hydrochloride (TCEP) are used to protect antibody protein from complicated cell lysate mixture.
- Protein purification by chromatography. Specific binding of the protein tags can be used in affinity chromatography to achieve strong and specific antibody protein.
In addition, SDS-PAGE analysis is used to determine the antibody protein yield from the small-scale expression. SDS-PAGE is also used to confirm the purification efficiency and purity of protein after each purification step.
What Can We Do For You?
Creative Biolabs has extensive expertise and research experience in the field of recombinant antibody production. We can produce various formats recombinant antibodies including full-length antibodies, scFv, Fab/Fabʹ, sdAb/VHH, Fc fusion proteins, and chimeric antibody. Customers only need to provide us a gene sequence or hybridoma cells. We also offer small-scale expression service for recombinant antibodies production with optimal expression system such as E.coli. If you are interested in our service, please contact us for more information.
Work flow of rAb production includes:
