Detection Assays
Creative Biolabs specializes in mammalian, bacterial, and insect cell-based recombinant protein expression and purification. We offer a broad range of tailored services for the development and production of recombinant antibodies, including various antibody formats. At present, our comprehensive detection assays for antibody products will guarantee antibodies meet your requirements, including Enzyme-linked immunosorbent assay (ELISA), Western Blot (WB), Immunohistochemistry (IHC), Immunofluorescence (IF), Flow cytometry (FC) analysis and so on.
Detection Assays for Antibody Production
Producing a dependable antibody and with predictable performance is a long process besides purification and clonal selection. In order to understand and anticipate how an antibody will perform and interact in application, antibody screening and characterization using suitable detection assays is fundamental.
Generally, customized detection assays for antibody validation include ELISA, WB, IHC, IF, FC, etc.
ELISA is one of the most commonly employed screening methods during detection assays for antibody production. The assay is first created by Engvall and Perlmann in 1971. The process is to use a solid-phase enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample based on antibodies directed against the ligand. ELISA presently has been used as an effective diagnostic tool in medicine, plant pathology, biotechnology and a quality control check in various industries. In short, antigens from the sample are attached to a surface. Then, a specific antibody that only recognizes the antigen is applied over the surface so it can bind to the antigen. Subsequently, a secondary antibody against the primary antibody is conjugated to an enzyme, and a substance containing the enzyme's substrate is added. The final reaction produces a detectable signal, most commonly a color change.
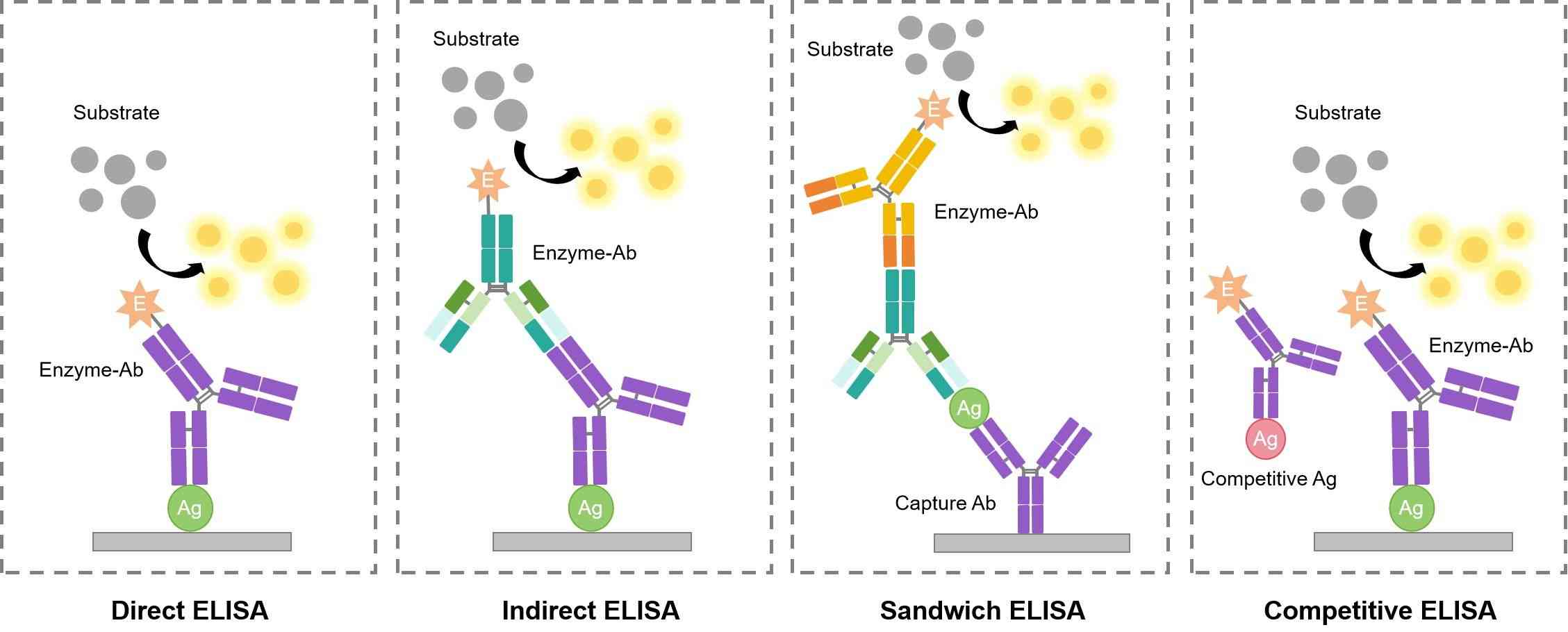 Fig.1 Different patterns of ELISA.
Fig.1 Different patterns of ELISA.
The western blot also known as western blotting or protein immunoblot, is a useful analytical technique widely used in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Simply, the samples were denatured and then by gel electrophoresis. The primary antibody is added that can specifically recognize and binds to a target protein. The electrophoretic membrane is washed in a solution containing the primary antibody, and then the excess antibody is washed. Subsequently, a secondary antibody against the primary antibody is added. Then, the secondary antibody is visualized through various methods such as staining, immunofluorescence, and radioactivity, allowing indirect detection of the specific target protein.
IHC is one of the most common assays for immunostaining. In theory, the method involves the process of selectively identifying antigens by using the antibodies that can specifically recognize and bind to antigens in biological tissues. It was described by Albert Coons conceptualized in 1941. IHC is widely used in the detection and diagnosis of abnormal cells such as cancerous tumors. It is also used in basic research to understand the distribution and location of biomarkers and differentially expressed proteins in different parts of biological tissues. The process of this assay is similar to WB or ELISA, mainly used antibodies that can specifically recognize antigens, and the indirect detection of the specific target protein by staining, immunofluorescence, etc.
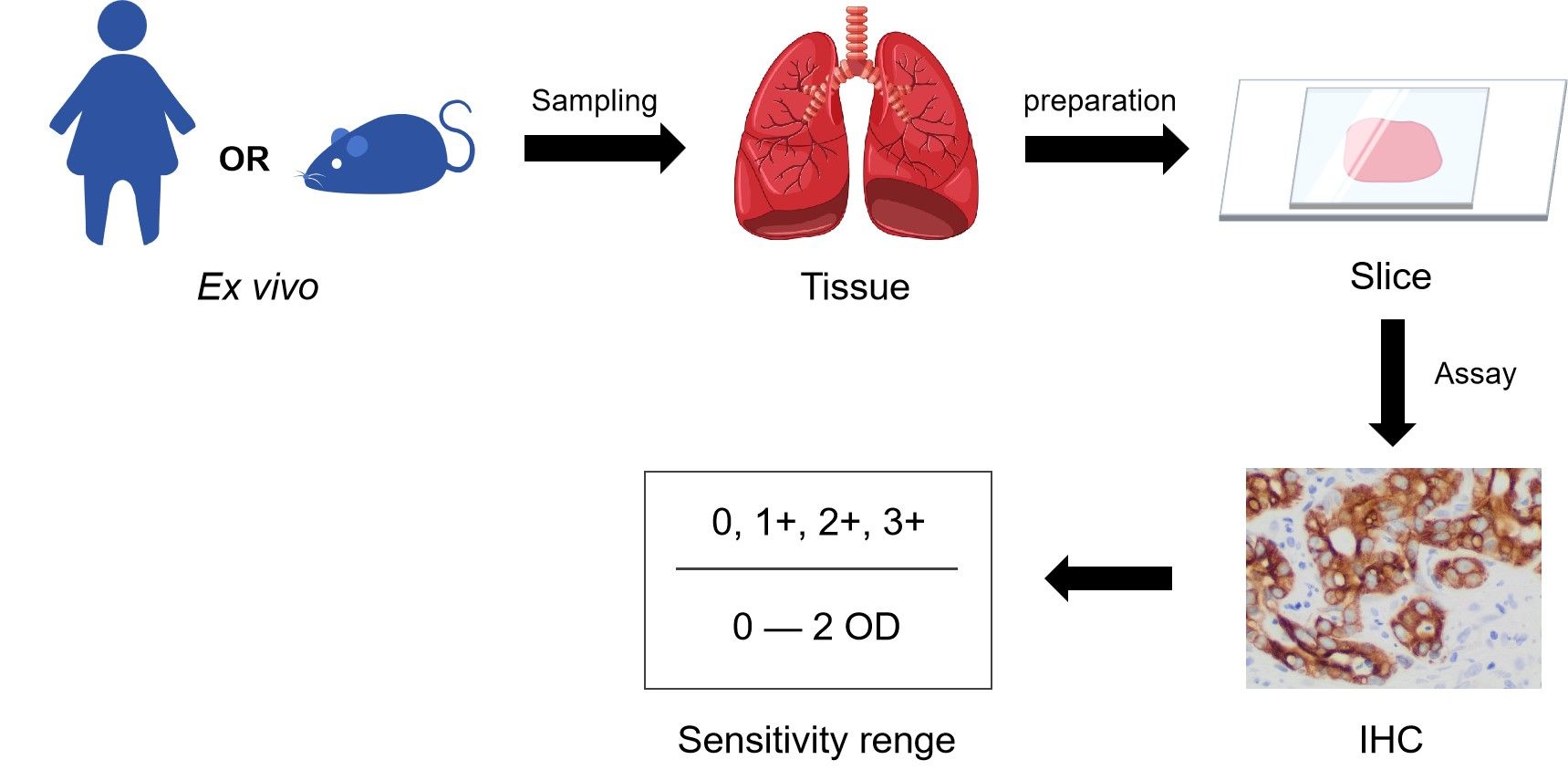 Fig.2 Scheme of the IHC.
Fig.2 Scheme of the IHC.
IF is a technique combined with light microscopy with a fluorescence microscope and is widely used for immunostaining. In brief, this method applies the antibodies that specifically bind to antigen, and to target fluorescent dyes to specific biomolecule targets within a cell. Thus, achieve the visualization of the distribution of the target molecule through the sample. Importantly, the conjugation of the fluorophore to the antibody itself cannot interfere with the immunological specificity of the antibody to target antigen. IF primarily makes use of fluorophores to visualize the location of the antibodies.
FC is a technique widely used in the detection and measurement of cells or particles physical and chemical characteristics. Theoretically, a sample containing cells or particles is suspended in a fluid and injected into the flow cytometer instrument. The sample is focused to ideally flow one cell at a time through a laser beam, where the light scattered is characteristic to the cells and their components. Then cells are labeled with fluorescent markers and light is absorbed to emit in a band of wavelengths. A large number of cells can be rapidly detected and the data gathered are processed by a computer. FC can be used in basic research, clinical practice, and clinical trials.
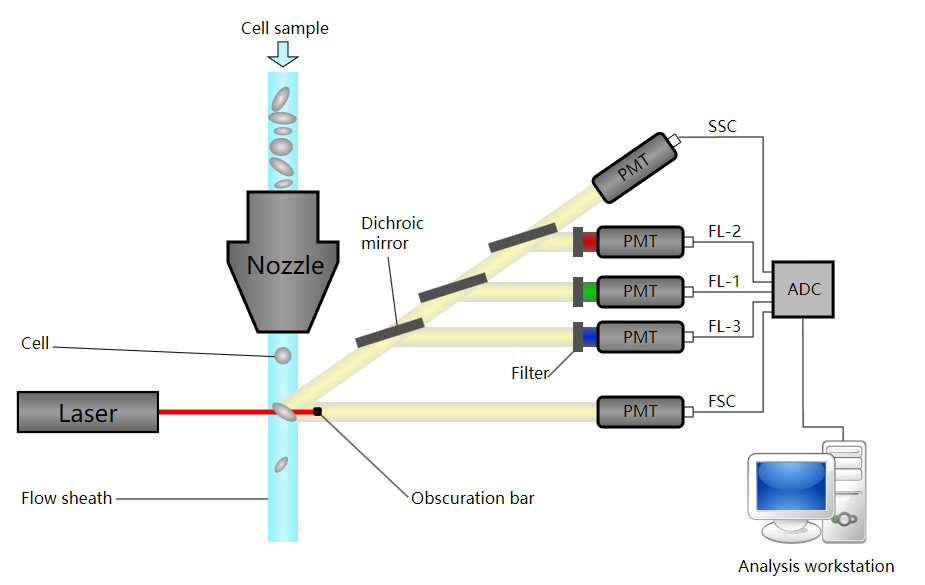 Fig.3 Schematic of a flow cytometer.1
Fig.3 Schematic of a flow cytometer.1
For more information such as the protocol about antibody detection assays, please contact us.
Creative Biolabs' years of expertise and propriety high throughput technologies allow us to offer a suite of detection assays services for antibody screening and quality control requirements. Best of all these services easily and seamlessly integrate with our custom antibody production services. If you are interested in our service, please do not hesitate to contact us for more details.
Work flow of rAb production includes:
- Gene Cloning
- Transformation of Expression Plasmids
- Small-scale Expression
- Large-scale Expression & Bacterial Disruption
- Purification of Fusion Proteins
- Protein Concentration & Concentration Determination
- Detection Assays
Reference
- From Wikipedia: By Kierano - Own work, CC BY 3.0, https://commons.wikimedia.org/wiki/File:Cytometer.svg
