Streptavidin Antibodies
Background
Streptavidin is a tetramer protein derived from Streptomycin, renowned for its extremely high affinity for biotin (Kd≈10^-14 M), and has become an indispensable tool in modern biotechnology. This protein is composed of four identical β -barrel subunits, each of which can specifically bind to a biotin molecule. This unique molecular recognition property has enabled it to be widely applied in fields such as immunoassay, molecular imaging, and targeted drug delivery. In 1990, the analysis of its crystal structure revealed a precise bonding pocket structure. Subsequent research further clarified the allosteric mechanism by which it achieves cooperative bonding through a water-mediated network. As a model of protein-ligand interaction, streptavidin not only provides an ideal model for molecular recognition research, but its engineered products also open up new avenues for diagnosis, treatment and nanotechnology.
Structure of Streptavidin
Streptavidin is a monomeric protein composed of 159 amino acids, forming a typical β -barrel-shaped three-dimensional structure through 8 reverse-parallel β -folded sheets (β1-β8), among which the β3-β4 ring region constitutes a specific biotin binding site. This site is composed of key residues such as Trp79, Trp92 and Asp128. Particularly, Trp120 forms a stable hydrophobic interaction with the biotin-imidazolone ring, endowing it with an extremely strong binding ability. The protein surface is rich in hydrophilic groups, which endows it with good water solubility. The tetramer subunits maintain structural stability through the hydrogen bond network between β -folded sheets, demonstrating excellent heat resistance (able to withstand temperatures up to 80 °C) and resistance to protease degradation. This unique structural feature makes it an indispensable tool protein in the field of biotechnology.
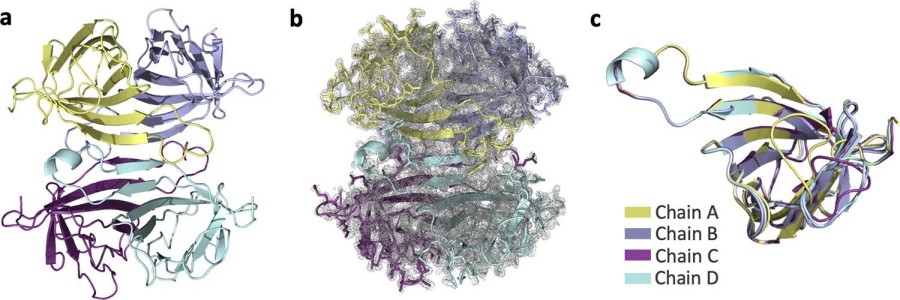 Fig. 1 Apo-SFX structure of streptavidin.1
Fig. 1 Apo-SFX structure of streptavidin.1
Key structural properties of Streptavidin:
- Tetramer β -barrel folded structure
- Each subunit contains a specific biotin binding site
- High-temperature resistant (Tm > 70℃) and extreme pH
- Surface charged residues affect solubility
Functions of Streptavidin
Streptavidin is a tetramer protein with a molecular weight of approximately 52-60kDa, and its monomer molecular weight is about 13-15kDa. This protein shows structural differences among different sources:
| Function | Description |
| Biotin binding | Each subunit can specifically bind to a biotin molecule, with a dissociation constant of 10^14 m, forming one of the strongest non-covalent bond interactions in nature. |
| Molecular anchoring | Widely used in biological molecules of fixed and detection, high sensitivity analysis of the immune. |
| Nanotechnology | As molecular "glue" for self-assembly of nanomaterials and biosensor construction. |
| Drug delivery | Targeted drug delivery and controlled release of drugs are achieved through the biotin-avidin system. |
| Diagnostic application | As a signal amplification system for ELISA, flow cytometry and other diagnostic techniques. |
Compared with antibody-antigen interactions, the streptavidin-biotin system exhibits unique binding kinetic characteristics: a rapid binding rate and an extremely slow dissociation rate, making it a model of irreversible binding. This characteristic gives it an irreplaceable advantage in molecular immobilization applications that require long-lasting stability.
Applications of Streptavidin and Streptavidin Antibody in Literature
1. Ayan, Esra, et al. "Cooperative allostery and structural dynamics of streptavidin at cryogenic-and ambient-temperature." Communications biology 5.1 (2022): 73. https://doi.org/10.1038/s42003-021-02903-7
This study resolved the high-resolution crystal structures of two non-bound (Apo) states of streptavidin (1.7 A at room temperature SFX and 1.1 A at low temperature freezing). Combined with computational analysis, it reveals that it forms a cooperative allosteric mechanism that simulates biotin binding through a water molecule network, providing new insights into the dynamic structure of this naturally strongest affinity system.
2. Suganuma, Masatoshi, et al. "Mirror-image streptavidin with specific binding to L-biotin, the unnatural enantiomer." Scientific Reports 12.1 (2022): 9568. https://doi.org/10.1038/s41598-022-13763-4
The research team successfully synthesized D-type streptavidin enantiomers, which can specifically bind to L-biotin. This artificial protein was prepared through the synthesis of all-D-amino acid peptides and in vitro folding, maintaining stability and structural characteristics comparable to those of natural streptavidin. This new type of streptavidin can effectively eliminate the interference of D-biotin in serum detection and shows superior performance in detection systems such as Biacore and ELISA, providing an innovative alternative to the biotin-streptavidin system.
3. Pähler, A., et al. "Characterization and crystallization of core streptavidin." Journal of Biological Chemistry 262.29 (1987): 13933-13937.https://doi.org/10.1016/S0021-9258(18)47884-2
The core streptavidin retains its complete biotin-binding activity after being truncated, its solubility is enhanced, and its crystal structure shows a D2-symmetric tetramer. Conformational changes occur when binding to biotin. Currently, the three-dimensional structure of its complex with selenobiotin is being resolved.
4. Zhang, Min, et al. "The crystal structure of monovalent streptavidin." Scientific reports 6.1 (2016): 35915. https://doi.org/10.1038/srep35915
This paper analyzed the 1.7A crystal structure of monovalent streptavidin (a single-site high-affinity binding biotin), and found that the L3,4 loops of its three failed subunits were in an "open state", and the hydrogen bond network involving S52 was stable. Mutations at key sites (N23A/S27D/S45A) further reduce biotin binding capacity by stabilizing the open state, providing a structural basis for rational design.
5. Nödling, Alexander R., et al. "The role of streptavidin and its variants in catalysis by biotinylated secondary amines." Organic & biomolecular chemistry 19.47 (2021): 10424-10431. https://doi.org/10.1039/D1OB01947C
This study combined host screening, crystallography and QM/MM simulation to explore the influence of streptavidin protein structure on the 1, 4-conjugated addition reaction catalyzed by biotinylated secondary amines. It was found that the tetramer T-Sav significantly enhanced the stereoselectivity of the reaction through subunit interface residues, among which Lys121 played a key role in controlling stereoselectivity and reactivity. Although the asymmetric dimer D-Sav has a relatively low conversion rate, it is more suitable for subsequent protein engineering optimization.
Creative Biolabs: Streptavidin Antibodies for Research
Creative Biolabs specializes in the production of high-quality Streptavidin antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom Streptavidin Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our Streptavidin antibodies, custom preparations, or technical support, please contact us.
Reference
- Ayan, Esra, et al. "Cooperative allostery and structural dynamics of streptavidin at cryogenic-and ambient-temperature." Communications biology 5.1 (2022): 73. https://doi.org/10.1038/s42003-021-02903-7
Anti-Streptavidin antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-ALK (Phosphorylated Y1278) Recombinant Antibody (D59G10) (PTM-CBMAB-0035YC)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0435) (CBMAB-0437-YY)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

-
Mouse Anti-ATP1B3 Recombinant Antibody (1E9) (CBMAB-A4021-YC)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Rabbit Anti-ABL1 (Phosphorylated Y185) Recombinant Antibody (V2-443434) (PTM-CBMAB-0001YC)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Mouse Anti-DLL4 Recombinant Antibody (D1090) (CBMAB-D1090-YC)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-CD247 Recombinant Antibody (6B10.2) (CBMAB-C1583-YY)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-1728) (CBMAB-2077-YY)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






