MIB1 Antibodies
Background
The MIB1 gene encodes an E3 ubiquitin ligase, which is mainly distributed in the cell nucleus and serves as a key regulatory factor of the Hippo signaling pathway, participating in the negative regulation of cell cycle progress and cell proliferation. This protein maintains genomic stability by mediating the ubiquitination degradation of specific substrates, and its dysfunction is closely related to the occurrence of various tumors and neurodevelopmental abnormalities. First identified in fruit fly research in 1996, MIB1 is a core regulatory element of the Notch signaling pathway. Subsequent studies have revealed that it plays multiple roles in neuronal development, immune response, and DNA damage repair. The complex functional network of this gene provides an important molecular basis for understanding cell fate determination and disease mechanisms, and has become an important research target in cancer biology and developmental medicine.
Structure of MIB1
MIB1 is an E3 ubiquitin ligase with a molecular weight of approximately 59.2 kDa. This protein is composed of 1,006 amino acids, forming a characteristic RING domain and multiple zinc finger domains. There are subtle differences in the molecular weight of MIB1 among different species:
| Species | Human | Mouse | Zebrafish | Fruit fly |
| Molecular Weight (kDa) | 59.2 | 58.9 | 57.8 | 54.6 |
| Primary Structural Differences | RING structure domain and 13 ANK repeats | 92% homology with humans | Retain the core functional domain | Simplified structure version |
The MIB1 protein exerts E3 ubiquitin ligase activity through its N-terminal RING domain, and the C-terminal ANK repeat mediates protein-protein interactions. This enzyme can recognize specific substrates and catalyze ubiquitination modification, playing a key role in the regulation of the Notch signaling pathway and the cell cycle process. Its highly conserved functional domains ensure that similar molecular mechanisms are maintained across different species.
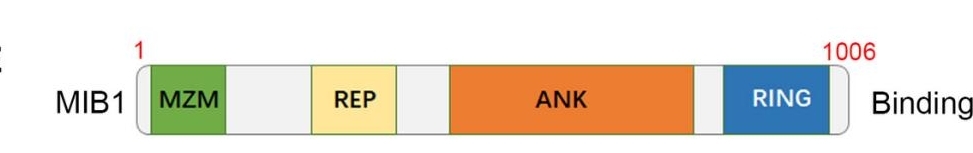 Fig. 1 A schematic diagram depicting the domain of MIB1.1
Fig. 1 A schematic diagram depicting the domain of MIB1.1
Key structural properties of MIB1:
- Contains the N-terminal RING domain
- Multiple ANK repeat sequences
- The zinc finger domain at the C-end
Functions of MIB1
The core function of MIB1 protein is to act as an E3 ubiquitin ligase to regulate cellular signal transduction. Its main physiological functions include:
| Function | Description |
| Notch signaling regulation | By ubiquitinating the Notch receptor ligand, it promotes the activation and endocytosis of the Notch signaling pathway and regulates cell fate determination. |
| Cell cycle regulation | Target key factors such as cyclin for ubiquitination and degradation to maintain the normal cell cycle process. |
| Neuronal development | In the neural regulation of axon guidance, synapse formation process influence the establishment of the neural circuits. |
| Immune response | By regulating the activity of signal molecules in immune cells, it participates in the moderate control of inflammatory responses. |
| Tumor suppression | By promoting the degradation of proto-oncoprotein and exerting tumor suppressive effects, its abnormal expression is associated with various cancers. |
MIB1 forms a complex regulatory network by recognizing specific substrates and catalyzing the synthesis of ubiquitin chains. Unlike the single-function E3 ligase, MIB1 can integrate multiple signaling pathways, which is of crucial significance in embryonic development and the maintenance of tissue homeostasis. Its functional diversity stems from the synergistic effect of multiple protein-protein interaction domains within itself.
Applications of MIB1 and MIB1 Antibody in Literature
1. Piñeiro-Sabarís, Rebeca, Donal MacGrogan, and José Luis de la Pompa. "Intricate MIB1-NOTCH-GATA6 Interactions in Cardiac Valvular and Septal Development." Journal of Cardiovascular Development and Disease 11.7 (2024): 223. https://doi.org/10.3390/jcdd11070223
Studies have shown that the MIB1 and GATA6 genes interact in congenital heart disease. The Mib1V943F mutation can inhibit the phenotype of bicuspid aortic valve and ventricular septal defect caused by Gata6 deletion. Functional analysis shows that this effect is related to the regulation of the epithelial-mesenchymal transition pathway, revealing the complex regulatory relationship between MIB1-NOTCH and GATA6 in cardiac development.
2. Zhang, Bin, et al. "MIB1 upregulates IQGAP1 and promotes pancreatic cancer progression by inducing ST7 degradation." Molecular Oncology 15.11 (2021): 3062-3075. https://doi.org/10.1002/1878-0261.12955
This study reveals a novel mechanism by which MIB1 drives the progression of pancreatic cancer by targeting and degrading the tumor suppressor protein ST7, thereby up-regulating IQGAP1. Elevated expression of MIB1 is associated with poor prognosis in patients, suggesting that it can serve as a potential new therapeutic target.
3. Li, Binbin, et al. "MIB1 mutations reduce Notch signaling activation and contribute to congenital heart disease." Clinical Science 132.23 (2018): 2483-2491. https://doi.org/10.1042/CS20180732
This study has for the first time discovered a novel and rare mutation of the MIB1 gene in patients with congenital heart disease. Among them, the p.T312K fs*55 and p.W271G mutations will weaken the function of MIB1, resulting in the decline of JAGGED1 ubiquitination mediated by it and the activation ability of the Notch signaling pathway, revealing that MIB1 mutations are a new genetic cause of congenital heart disease.
4. Wang, Pengtao, et al. "SNX17 recruits USP9X to antagonize MIB1-mediated ubiquitination and degradation of PCM1 during serum-starvation-induced ciliogenesis." Cells 8.11 (2019): 1335. https://doi.org/10.3390/cells8111335
This study found that under serum starvation conditions, SNX17 antagonized the ubiquitination and degradation of the centriomeric satellite protein PCM1 by MIB1 by recruiting the deubiquitinating enzyme USP9X, thereby maintaining the stability of this protein and ensuring normal ciliary occurrence. This mechanism reveals a new potential cause of diseases related to USP9X defects.
5. Zhu, Ziman, et al. "A novel long-noncoding RNA LncZFAS1 prevents MPP+-induced neuroinflammation through MIB1 activation." Molecular Neurobiology 59.2 (2022): 778-799. https://doi.org/10.1007/s12035-021-02619-z
This study reveals a new role of MIB1 in Parkinson's disease models. MIB1 inhibits the activation of the NLRP3 inflammasome by mediating the ubiquitination of the TXNIP protein. Under the induction of MPP+, the upregulation of miR590-3p expression will interfere with the function of MIB1, thereby activating the inflammatory response. LncZFAS1 can maintain the protective effect of MIB1 by inhibiting miR590-3p.
Creative Biolabs: MIB1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality MIB1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MIB1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MIB1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Zhang, Bin, et al. "MIB1 upregulates IQGAP1 and promotes pancreatic cancer progression by inducing ST7 degradation." Molecular Oncology 15.11 (2021): 3062-3075. https://doi.org/10.1002/1878-0261.12955
Anti-MIB1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-BIRC5 Recombinant Antibody (6E4) (CBMAB-CP2646-LY)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-CFL1 Recombinant Antibody (CBFYC-1771) (CBMAB-C1833-FY)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Mouse Anti-FN1 Monoclonal Antibody (D6) (CBMAB-1240CQ)

-
Mouse Anti-BHMT Recombinant Antibody (CBYY-0547) (CBMAB-0550-YY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Rabbit Anti-ALK (Phosphorylated Y1278) Recombinant Antibody (D59G10) (PTM-CBMAB-0035YC)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-CTCF Recombinant Antibody (CBFYC-2371) (CBMAB-C2443-FY)

-
Rabbit Anti-ABL1 (Phosphorylated Y185) Recombinant Antibody (V2-443434) (PTM-CBMAB-0001YC)

-
Mouse Anti-AP4E1 Recombinant Antibody (32) (CBMAB-A2996-YC)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-BACE1 Recombinant Antibody (61-3E7) (CBMAB-1183-CN)

-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








