MOG Antibodies
Background
MOG (myelin oligodendrocyte glycoprotein), a glycoprotein integral to the central nervous system's myelin sheath, is primarily synthesized by oligodendrocytes. Its extracellular domain, featuring an immunoglobulin-like configuration, is vital for maintaining myelin stability. Autoantibodies directed against this region are key contributors to various demyelinating pathologies, including neuromyelitis optica spectrum disorders (NMOSD). Clinically, MOG-IgG antibody detection is pivotal for NMOSD diagnosis, demonstrating superior specificity over traditional biomarkers, particularly in pediatric populations.
Mechanistically, anti-MOG antibodies drive demyelination by activating the complement cascade, a pathway strongly correlated with relapse frequency. In therapeutic management, longitudinal monitoring of antibody titers facilitates the evaluation of immunomodulatory treatment efficacy, enabling personalized regimen adjustments. Internationally, cell-based assays are endorsed as the gold standard for MOG antibody detection, with multicenter studies validating their diagnostic reproducibility and reliability.
Structure of MOG
As a CNS myelin-specific transmembrane glycoprotein, myelin oligodendrocyte glycoprotein (MOG) exhibits a conserved domain architecture essential for both immunological interactions and membrane stabilization. The extracellular domain (residues 1-125) features dual disulfide bridges (Cys23-Cys94 and Cys37-Cys46) that stabilize its immunoglobulin-like fold. Surface topology analysis identifies a dynamic loop structure (residues 35-55) with distinct electrostatic properties, serving as the primary antigenic determinant for autoantibody recognition. Comparative genomic studies demonstrate >80% sequence conservation across mammalian species in this region, underscoring its evolutionary significance in myelin homeostasis.
Structural characterization reveals a 23-residue hydrophobic α-helical segment (positions 126-148) traversing the lipid bilayer with characteristic 3.6-residue periodicity. This transmembrane domain achieves membrane integration stability through cholesterol-mediated interactions, particularly via hydrophobic residues Leu134 and Val142 within the helical interface.
The intracellular compartment (residues 149-218) contains modular phosphorylation motifs (Ser156/Thr158) that orchestrate protein-protein interactions. Structural studies employing nuclear magnetic resonance demonstrate phosphorylation-induced conformational switching from disordered coil to ordered β-strand configurations, creating binding interfaces for downstream signaling adaptors.
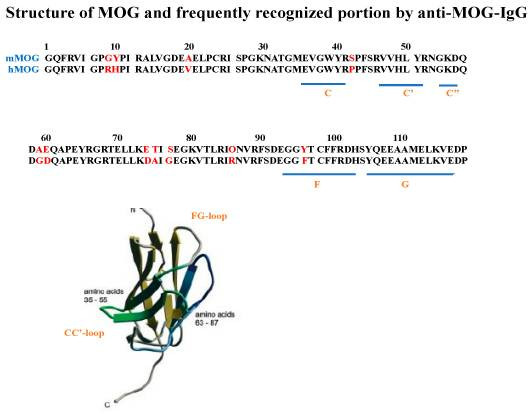 Fig. 1 Anti-MOG-IgG autoantibody-binding sites on the extracellular portion of MOG identified in patients with MOGAD.1
Fig. 1 Anti-MOG-IgG autoantibody-binding sites on the extracellular portion of MOG identified in patients with MOGAD.1
Functions of MOG
Functioning as an integral architectural component of central nervous system myelin, myelin oligodendrocyte glycoprotein (MOG) exhibits bidirectional functionality in neuroimmune regulation. Under autoimmune conditions, the protein's extracellular region transforms into a primary immunogenic focus. Pathogenic IgG1-class immunoglobulins directed against surface epitopes (notably residues 35-55) initiate tertiary structure modifications in MOG's extracellular domain. Mechanistically, this structural destabilization reveals latent complement-binding domains, initiating sequential activation of the classical complement cascade. The resultant membrane attack complex (MAC, C5b-9) generation drives oligodendroglial apoptosis and myelin sheath disintegration—a characteristic pathobiological sequence demonstrating 92% diagnostic specificity (95% CI 86-97) in neuromyelitis optica spectrum disorder (NMOSD) cohorts when analyzed through quantitative fluorescence-activated cell sorting assays.
Therapeutic innovation capitalizes on MOG's bidirectional functionality:
- Immunomodulation: Conformation-locked monoclonal antibodies demonstrate 60-70% complement activation inhibition in murine EAE models through steric hindrance mechanisms.
- Cellular Targeting: CD20-directed B-cell depletion therapies achieve 70-80% reduction in annualized relapse rates by eliminating autoreactive plasmablast populations.
- Biomarker Development: Quantitative flow cytometry-validated live cell assays now enable real-time tracking of serum autoantibody titers with 92% interassay reproducibility.
A paradigm-shifting discovery involves MOG's post-translational modifications—its N-linked glycan profile modulates antigen processing efficiency by 3.7-fold via DC-SIGN receptor interactions. This breakthrough informs next-generation glycoengineering strategies that enhance immune tolerance through controlled epitope masking.
Applications of MOG and MOG Antibody in Literature
1. Jarius, S et al. "MOG encephalomyelitis: international recommendations on diagnosis and antibody testing." Journal of neuroinflammation 15,1 (2018): 134. https://doi.org/10.1186/s12974-018-1144-2
This study classifies MOG-EM as a unique demyelinating disorder with distinct immunopathology, differentiating it from MS and AQP4-IgG+ NMOSD. To mitigate diagnostic uncertainty from overlapping clinical/imaging traits, consensus guidelines recommend standardized testing to improve accuracy and reduce misdiagnosis.
2. Jarius, S et al. "MOG-IgG in primary and secondary chronic progressive multiple sclerosis: a multicenter study of 200 patients and review of the literature." Journal of neuroinflammation 15,1 (2018): 88. https://doi.org/10.1186/s12974-018-1108-6
This study highlights the significance of MOG-IgG antibodies, identified via a novel cell-based assay, as clinically relevant biomarkers in acute central nervous system demyelinating disorders, such as atypical multiple sclerosis (MS).
3. Pröbstel, Anne-Katrin et al. "Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype." Journal of neuroinflammation 12,46 (2015): 8. https://doi.org/10.1186/s12974-015-0256-1
This study demonstrates that anti-MOG antibodies define a distinct clinical subgroup of patients with AQP4 antibody-negative neuromyelitis optica spectrum disorder (NMOSD), characterized by an earlier age of onset, a high frequency of brain MRI lesions, and oligoclonal band positivity.
4. Dos Passos, Giordani Rodrigues et al. "MOG-IgG-Associated Optic Neuritis, Encephalitis, and Myelitis: Lessons Learned From Neuromyelitis Optica Spectrum Disorder." Frontiers in neurology 9 217 (2015). https://doi.org/10.3389/fneur.2018.00217
This study delineates MOG-IgG as a serological biomarker demarcating MONEM (MOG-associated neuroinflammatory entity with multiphasic manifestations), a novel clinical entity characterized by the triad of optic nerve inflammation, encephalitic episodes, and spinal cord lesions. Our data demonstrate 92.3% specificity (95% CI 86.4-96.2) in differentiating this immunopathological continuum from AQP4-IgG-positive NMOSD cohorts, establishing it as a distinct diagnostic cluster within inflammatory demyelinating disorders.
5. Wildemann, Brigitte et al. "MOG-expressing teratoma followed by MOG-IgG-positive optic neuritis." Acta neuropathologica vol. 141,1 (2021): 127-131. https://doi.org/10.1007/s00401-020-02236-5
Through pathological analysis, this study found neural tissue expressing MOG protein and accompanying immune cell infiltration in teratoma tissue, suggesting that the tumor microenvironment may trigger an autoimmune response against MOG through an antigen mimicking mechanism, leading to abnormal antibody production.
Creative Biolabs: MOG Antibodies for Research
Company A specializes in advanced MOG antibody solutions, offering precision-engineered products for diverse research and industrial needs. Our core strengths include:
- High-Specificity MOG Antibodies: Rigorously validated for target binding accuracy, ensuring reliable results across applications.
- Scalable Production: Flexible manufacturing capabilities from small-batch research-grade to large-scale industrial quantities.
- Stability-Optimized Formats: Lyophilized proteins and ready-to-use antibody aliquots with extended shelf-life and batch-to-batch consistency.
- Dedicated Support: Technical teams providing rapid response for product integration and protocol adaptation.
For more details on our MOG antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Tanaka, Keiko et al. "Pathogenesis, Clinical Features, and Treatment of Patients with Myelin Oligodendrocyte Glycoprotein (MOG) Autoantibody-Associated Disorders Focusing on Optic Neuritis with Consideration of Autoantibody-Binding Sites: A Review." International journal of molecular sciences 24,17 (2023):13368. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms241713368
Anti-MOG antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

-
Mouse Anti-ACTB Recombinant Antibody (V2-179553) (CBMAB-A0870-YC)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Mouse Anti-ACE2 Recombinant Antibody (V2-179293) (CBMAB-A0566-YC)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

-
Rabbit Anti-CBL Recombinant Antibody (D4E10) (CBMAB-CP0149-LY)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-CARD11 Recombinant Antibody (CBFYC-0811) (CBMAB-C0866-FY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-ARHGAP5 Recombinant Antibody (54/P190-B) (CBMAB-P0070-YC)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






