ERH Antibodies
Background
ERH is a conserved small molecule protein, mainly existing in the nucleus of eukaryotes, and is involved in gene expression regulation and the cell cycle process. This protein plays a key role in the process of pre-mRNA splicing and transcriptional extension by interacting with mRNA splicing factors and transcriptional extension complexes, thereby influencing a variety of biological functions. Studies have found that ERH is abnormally expressed in various cancers and may be related to tumorigenesis and development. The ERH protein was initially identified for its function in Drosophila embryonic development. Its highly conserved three-dimensional structure consists of four β -folds and one α -helix. This simple and stable structure makes it an ideal model for studying protein-nucleic acid interactions. In recent years, scientists have analyzed the structural features of ERH through techniques such as X-ray crystallography and nuclear magnetic resonance, providing important clues for understanding its molecular mechanism in the gene expression regulatory network.
Structure of ERH
ERH is a relatively small nucleoprotein with a molecular weight of approximately 12-14 kDa, and there are subtle differences among different species:
| Species | Human | Mice | Fruit flies | Yeast |
| Molecular Weight (kDa) | 12.4 | 12.3 | 13.1 | 14.0 |
| Primary Structural Differences | Highly conservative, containing typical ERH domains | Highly homologous to human ERH | Participate in the regulation of RNA splicing | Play an important role in transcription extension |
ERH proteins are typically composed of 104 to 120 amino acids, forming a compact β -folded -α helical structure. Its tertiary structure is mainly composed of four β -folded lamellae and one C-terminal α -helix, forming a stable nucleic acid binding interface. This protein maintains structural stability through a conserved hydrophobic core and interacts with RNA or proteins by means of positively charged residues on its surface, participating in the regulation of gene expression. ERH is highly conserved in evolution, and its structural features enable it to flexibly participate in the assembly of various molecular complexes.
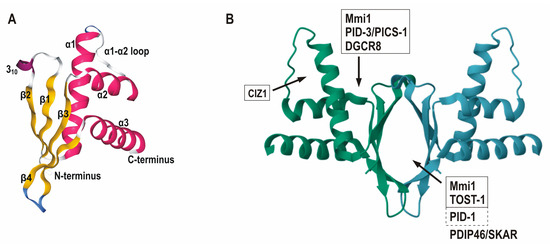 Fig. 1 Three-dimensional structure of ERH and its protein partner binding sites. 1
Fig. 1 Three-dimensional structure of ERH and its protein partner binding sites. 1
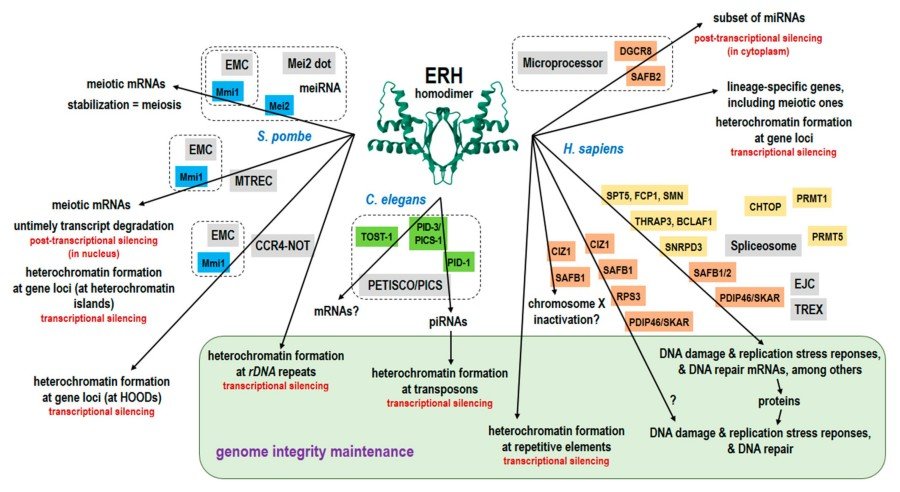 Fig. 2 Diagrammatic summary of the ERH functions, including the proposed genome integrity maintenance.1
Fig. 2 Diagrammatic summary of the ERH functions, including the proposed genome integrity maintenance.1
Key structural properties of ERH:
- Conservative β -folded -α helical structure
- Hydrophobic core to maintain stability
- Surface of positively charged molecules
- Conservative dimerization interface
- Flexible connection area
Functions of ERH
The main function of ERH protein is to participate in the regulation of gene expression and play a key role in various cellular processes:
| Function | Description |
| Regulation of pre-mRNA splicing | By interacting with splicing factors, it regulates the alternative splicing process of mRNA. |
| Regulation of transcriptional extension | As a component of the transcriptional extension complex, it affects the progression of RNA polymerase II. |
| Cell cycle regulation | By regulating the expression of key genes, it affects the activation of cell cycle checkpoints. |
| DNA damage response | Participate in DNA damage repair pathways and maintain genomic stability. |
| Cancer-related functions | Abnormal expression in various tumors may affect the transcriptional regulation of oncogenes. |
ERH participates in the regulatory network in a dose-dependent manner through its conserved protein-protein interaction interface (unlike the synergistic effect of hemoglobin/myoglobin), a characteristic that makes it a key molecule for the precise regulation of gene expression.
Applications of ERH and ERH Antibody in Literature
1. Kozlowski, Piotr. "Thirty years with ERH: an mRNA splicing and mitosis factor only or rather a novel genome integrity protector?." Cells 12.20 (2023): 2449. https://doi.org/10.3390/cells12202449
The article indicates that ERH is a highly conserved nucleoprotein that forms a homodimer scaffold, participates in the metabolism of RNA Pol II transcripts (mRNA and ncRNA processing) and heterochromatin silencing, and maintains genomic stability through interaction with species-specific proteins (such as Schizoyeast Mmi1, human DGCR8, etc.).
2. Xie, Guodong, et al. "A conserved dimer interface connects ERH and YTH family proteins to promote gene silencing." Nature communications 10.1 (2019): 251.https://doi.org/10.1038/s41467-018-08273-9
The article indicates that Erh1 (human ERH homolog) in Schizoyeast forms a 2:2 complex (EMC) with Mmi1, and its eutectic structure reveals a conserved binding interface. The Mmi1Trp112 mutation disrupts heterochromatin assembly and gene silencing, but does not affect transcriptional termination, revealing a key interface for the interaction between ERH and RNA-binding proteins.
3. Banko, Monika I., et al. "Identification of amino acid residues of ERH required for its recruitment to nuclear speckles and replication foci in HeLa cells." PLoS One 8.8 (2013): e74885.https://doi.org/10.1371/journal.pone.0074885
The article indicates that ERH, as a unique small nucleoprotein, forms homodimers through the β -sheet surface. It interacts with PDIP46/SKAR to locate nuclear plaques (monomer form is required), and interacts with Ciz1 to locate replication foci (either monomer or dimer is acceptable). The key mutants (H3A Q9A/E37A T51A) reveal the separation of their localization mechanisms, providing a tool for studying their nuclear functions.
4. Pang, Kun, et al. "The ERH gene regulates migration and invasion in 5637 and T24 bladder cancer cells." BMC cancer 19.1 (2019): 225. https://doi.org/10.1186/s12885-019-5423-9
Research has found that knocking down the ERH gene can significantly inhibit the migration and invasion ability of bladder cancer T24/5637 cells (in vitro experiments and nude mouse models). Mechanism studies have shown that ERH affects tumor metastasis by regulating MYC gene expression, suggesting that ERH may become a new therapeutic target for bladder urothelial carcinoma.
5. Krzyzanowski, Marek K., Ewa Kozlowska, and Piotr Kozlowski. "Identification and functional analysis of the erh1+ gene encoding enhancer of rudimentary homolog from the fission yeast Schizosaccharomyces pombe." PloS one 7.11 (2012): e49059. https://doi.org/10.1371/journal.pone.0049059
Research has found that all four species of the genus Schizomyces contain ERH homologous genes, and the nucleoprotein Erh1 encoded by them responds to nutritional stress and hypertonic stress. erh1 deficiency leads to abnormal cell morphology, G1 phase arrest and enhanced stress sensitivity, but human ERH can partially compensate for these functions, suggesting its role in environmental adaptation and cell cycle regulation.
Creative Biolabs: ERH Antibodies for Research
Creative Biolabs specializes in the production of high-quality ERH antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ERH Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ERH antibodies, custom preparations, or technical support, contact us at email.
Reference
- Kozlowski, Piotr. "Thirty years with ERH: an mRNA splicing and mitosis factor only or rather a novel genome integrity protector?." Cells 12.20 (2023): 2449. https://doi.org/10.3390/cells12202449
Anti-ERH antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ALDOA Recombinant Antibody (A2) (CBMAB-A2316-YC)

-
Mouse Anti-CD24 Recombinant Antibody (ALB9) (CBMAB-0176CQ)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-ATP5F1A Recombinant Antibody (51) (CBMAB-A4043-YC)

-
Mouse Anti-AMACR Recombinant Antibody (CB34A) (CBMAB-CA034LY)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Rat Anti-CD300A Recombinant Antibody (172224) (CBMAB-C0423-LY)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Mouse Anti-DLG1 Monolconal Antibody (4F3) (CBMAB-0225-CN)

-
Mouse Anti-AKR1B1 Antibody (V2-2449) (CBMAB-1001CQ)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-CEMIP Recombinant Antibody (3C12) (CBMAB-K0296-LY)

-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-CCND2 Recombinant Antibody (DCS-3) (CBMAB-G1318-LY)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Mouse Anti-ADAM29 Recombinant Antibody (V2-179787) (CBMAB-A1149-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






