SF1 Antibodies
Background
The SF1 gene encodes a key nucleoprotein, which mainly participates in the splicing process of precursor mRNA and functions as a core component of the spliceosome complex. This gene affects the generation of various tissue-specific transcripts by regulating selective splicing patterns, and plays a particularly important role in the development of the reproductive and nervous systems. After being first identified by the Krainer team in 1991, SF1 has become a classic model for studying RNA-protein interactions due to its unique RNA recognition domain (KH domain) and zinc finger structure. Scientists have discovered that mutations in this gene are closely related to congenital developmental abnormalities and certain cancers. The precise molecular mechanism research has provided new ideas for targeted therapy, and the related achievements have been applied in the fields of genetic disease diagnosis and anti-cancer drug development.
Structure of SF1
Splicing factor 1 encoded by the SF1 gene is a nucleoprotein with a molecular weight of approximately 34-36 kDa, and its precise molecular weight varies slightly among different species.
| Species | Human | Mouse | Fruit fly | Nematode |
| Molecular Weight (kDa) | 35.2 | 34.8 | 36.1 | 33.9 |
| Primary Structural Differences | Contains KH domain and zinc finger motif | Highly conservative | Similar in function but with significant sequence differences | Simplified splicing factor |
The SF1 protein is composed of approximately 300 amino acids, and its three-dimensional structure contains two key functional domains: the KH (K homology) domain at the N-terminal is responsible for recognizing RNA sequences, while the zinc finger structure at the C-terminal participates in protein-protein interactions. This protein forms a stable RNA binding interface through the α -helix and β -folding in its secondary structure, in which the conserved GGNG motif directly binds to the pre-mRNA branch site. SF1 plays a scaffold role in the early stage of spliceosome assembly, and its conformational changes can regulate splicing efficiency. However, mutations in certain key amino acids (such as Arg240 and Lys303) can lead to splicing errors and are associated with various genetic diseases.
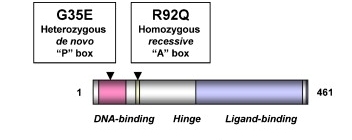 Fig. 1 Cartoon of SF-1 showing key functional domains and the position of the two SF-1 variants associated with this phenotype.1
Fig. 1 Cartoon of SF-1 showing key functional domains and the position of the two SF-1 variants associated with this phenotype.1
Key structural properties of SF1:
- KH domain with zinc finger motif
- Conserved RNA binding interface
- Dynamic conformation regulation
- Key functional sites
Functions of SF1
The core function of the SF1 gene is to regulate pre-mRNA splicing and participate in the regulation of multiple gene expression processes simultaneously.
| Function | Description |
| Spliceosome assembly | As the anchoring protein of U2 snRNP, it recognizes the branch site UACUAAC sequence and initiates the formation of the spliceosome A complex. |
| Selective splicing regulation | By regulating exon skipping or retention through variable binding strength, the formation of tissue-specific isomers is affected. |
| Transcriptional extension coupling | Interact with the Pol II CTD domain to coordinate the spatiotemporal coupling of transcription and splicing. |
| Non-classical editing supervision | Inhibit the use of abnormal concealed splicing sites and maintain the integrity of the mRNA coding framework. |
| Regulation of stress response | Reprogramming the splicing pattern under heat shock conditions promotes the expression of stress-related genes. |
The binding curve of the SF1 and U2AF complex is synergistic (nonlinear), reflecting its characteristic of dynamically regulating splicing efficiency through conformational changes. The latest research in "Nature Structural & Molecular Biology" reveals that the phosphorylation state of SF1 can change its RNA binding affinity and achieve differential splicing regulation during the cell cycle.
Applications of SF1 and SF1 Antibody in Literature
1. Wang, Xinyue, et al. "A novel rabbit anti-myoglobin monoclonal antibody's potential application in rhabdomyolysis associated acute kidney injury." International Journal of Molecular Sciences 24.9 (2023): 7822. https://doi.org/10.1007/s00401-024-02686-1
This study analyzed 270 cases of growth hormone-cell pituitary neuroendocrine tumors (PitNETs) and found that 74% of dense granular type (DGST) simultaneously expressed PIT1/SF1, and there were significant differences in clinicopathological and molecular characteristics compared with simple PIT1-positive DGST. The research suggests that such tumors co-expressing PIT1/SF1 should be classified separately as "growth gonadotropin cells PitNET", and it is recommended to revise the WHO classification standard.
2. Li, Xue, et al. "CXCR4-SF1 bifunctional adipose-derived stem cells benefit for the treatment of Leydig cell dysfunction-related diseases." Journal of Cellular and Molecular Medicine 24.8 (2020): 4633-4645. https://doi.org/10.1111/jcmm.15128
In this study, bifunctional adipose-derived stem cells (CXCR4-SF1-ADscs) expressing both CXCR4 and SF1 were constructed, which can be directed to migrate to the damaged testicles and differentiate into Leydig-like cells to repair BPA-induced Leydig cell dysfunction. Research has found that SF1 promotes testosterone synthesis by regulating the BSPRY gene, providing a new strategy for the treatment of Leydig cell dysfunction.
3. Godavarthi, Jyotsna D., et al. "Deficiency of Splicing Factor 1 (SF1) reduces intestinal polyp incidence in ApcMin/+ Mice." Biology 9.11 (2020): 398. https://doi.org/10.3390/biology9110398
Research has found that a reduction in the level of splicing factor SF1 can inhibit the formation of intestinal polyps in mice. In ApcMin/+ mutant mice, SF1 heterozygous deletion (Sf1+/−) reduced the number of polyps by 25-30%, especially in females and small polyps (≤2mm), suggesting that targeted reduction of SF1 may become a new strategy for preventing intestinal tumors.
4. Crisci, Angela, et al. "Mammalian splicing factor SF1 interacts with SURP domains of U2 snRNP-associated proteins." Nucleic acids research 43.21 (2015): 10456-10473. https://doi.org/10.1093/nar/gkv952
Studies have found that the splicing factor SF1 binds to the U2 snRNP protein through its conserved SURP interaction domain, recruiting U2 snRNP in the early formation of the spliceosome E complex. Experiments have confirmed that SF1 can pre-protrusion branch point sequences, promoting U2 snRNA pairing, and U2AF65 subsequently stabilizes this binding. The absence of SF1 reduces the formation of the A complex, suggesting its crucial role in the regulation of alternative splicing.
5. Barnea, Efrat, and Yehudit Bergman. "Synergy of SF1 and RAR in Activation of Oct-3/4Promoter." Journal of Biological Chemistry 275.9 (2000): 6608-6619. https://doi.org/10.1074/jbc.275.9.6608
Studies have found that SF1 is co-expressed with the embryonic transcription factor Oct-3/4 in P19 embryonic cancer cells and is simultaneously downregulated during retinoic acid-induced differentiation. SF1 recognizes two sites on the Oct-3/4 promoter through its DNA-binding domain (SF1(a) is located within RAREoct), and forms a novel complex with the retinoic acid receptor to synergistically activate the Oct-3/4 promoter, revealing for the first time the mechanism by which SF1 regulates embryonic specific genes.
Creative Biolabs: SF1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality SF1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom SF1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our SF1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Ferraz-de-Souza, Bruno, Lin Lin, and John C. Achermann. "Steroidogenic factor-1 (SF-1, NR5A1) and human disease." Molecular and cellular endocrinology 336.1-2 (2011): 198-205. https://doi.org/10.1016/j.mce.2010.11.006
Anti-SF1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Rat Anti-AChR Recombinant Antibody (V2-12500) (CBMAB-0990-CN)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-COL1A2 Recombinant Antibody (CF108) (V2LY-1206-LY626)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Mouse Anti-CHRNA9 Recombinant Antibody (8E4) (CBMAB-C9161-LY)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

-
Mouse Anti-CD33 Recombinant Antibody (6C5/2) (CBMAB-C8126-LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

-
Mouse Anti-NSUN6 Recombinant Antibody (D-5) (CBMAB-N3674-WJ)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Rabbit Anti-Acetyl-Histone H3 (Lys36) Recombinant Antibody (V2-623395) (CBMAB-CP0994-LY)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






