Biotin Conjugation
Biotin is frequently used in two-step detection systems in concert with conjugated avidin. Biotinylation is the process of attaching biotin to proteins and other macromolecules. Biotinylation reagents are available for targeting specific functional groups or residues, including primary amines, sulfhydryls, carboxyls and carbohydrates. The most common biotinylation reagent is an N-hydroxysuccimide (NHS)-activated biotin, which reacts with primary amine groups from the side chain of Lysine residues and the N-terminus of each polypeptide chain in the antibody.
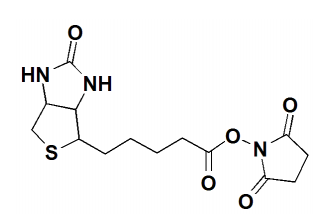 Fig.1 The structure of NHS-Biotin.
Fig.1 The structure of NHS-Biotin.
Protocol for Biotinylation with NHS-Biotin
This is a protocol for biotinylation of antibodies based on standard methods. For technical specifications and further details on the biotinylation, please follow the instructions provided by the chosen manufacturer’s biotinylation reagents.
Note: If commercially available purification columns are being used, please refer to the manufacturer's instructions for use.
Methods
- Allow vial of NHS-Biotin to equilibrate to ambient temperature before opening.
- Dissolve protein at a concentration of 1-10 mg/mL in 100 mM sodium phosphate, 150 mM NaCl, pH 7.2-7.5 or other suitable amine-free buffer.
- Immediately before use, create a 20 mg/mL NHS-Biotin stock solution in anhydrous DMF or DMSO.
- Add sufficient NHS-Biotin stock solution to the protein solution to obtain 10-20 fold molar excess of biotinylation reagent over protein.
- Allow biotinylation reaction to proceed for 30-60 minutes at RT or at least 2 h at 4°C.
- Dialyze 24-48 h against 1x PBS or desalt biotinylated protein through gel filtration with a resin or equivalent.
Important Notes on Biotinylation of Antibodies
- In some antibodies, it is possible that the Lysine residues are essential for antigen binding. In these cases, antigen-binding is reduced or totally lost with biotinylation. To overcome this issue, it is possible to reduce the amount of NHS-Biotin used. Alternatively, other chemistries such as carbohydrate coupling with a biotin hydrazide may be used.
- NHS-activated biotins react efficiently with primary amino groups in pH 7-9 buffers to form stable amide bonds. Generally, PBS pH 7.4 or 100 mol/L carbonate buffer with pH 8.0 or pH 8.4 are used for the biotinylation of antibodies, but the pH can be optimized further if required.
- Since NHS reacts with amine groups, these must not be present in the buffer. The antibody must not be in Tris buffer or contain sodium azide (NaN3), since they will block conjugation.
Troubleshooting
| Possible Cause | Recommended Solution |
| Low labeling rate |
The protein to be coupled was in a buffer containing primary amino groups or sugars
|
Other Protocols
Antibody Staining of Whole Mount Drosophila Embryos
