Cancer Immunotherapy Targeting Ferroptosis
Ferroptosis represents a unique, iron-dependent form of cell death driven by the accumulation of lipid peroxides. Traditional cancer treatments often face challenges such as drug resistance and low response rates. However, a deeper understanding of the complex interplay between ferroptosis and the tumor immune microenvironment is unlocking powerful new strategies.
The Mechanisms of Ferroptosis
Ferroptosis is a distinct form of programmed cell death characterized by metabolic dysregulation, oxidative stress, and iron-dependent lipid peroxidation. Unlike apoptosis, it lacks typical morphological features like chromatin condensation but is defined by mitochondrial changes such as membrane density increase and a smaller size. Understanding the core biochemical pathways is crucial for therapeutic intervention.
Iron Metabolism
Iron is a fundamental component of ferroptosis. The process is initiated by the accumulation of unstable ferrous iron (Fe2+) in the labile iron pool (LIP). This iron overload acts as a catalyst for the Fenton reaction, which converts hydrogen peroxide (H2O2) into highly reactive hydroxyl radicals (OH−). These radicals initiate a chain reaction of lipid peroxidation. Iron uptake is regulated by the transferrin-transferrin receptor (Tf-TFRC) complex, which imports ferric iron (Fe3+) into the cell for subsequent reduction. Conversely, the degradation of iron-storing ferritin by nuclear receptor co-activator 4 (NCOA4) via a process called ferritinophagy is a key pathway for increasing the intracellular LIP, thereby promoting ferroptosis.
Lipid Peroxidation
The direct executor of ferroptosis is the uncontrolled peroxidation of polyunsaturated fatty acids (PUFAs) on the cell membrane. This process is primarily mediated by the ACSL4-LPCAT3-lipoxygenase (ALOX) axis. Specifically, the enzyme acyl-CoA synthetase long-chain family member 4 (ACSL4) activates PUFAs, which are then incorporated into phospholipids. The subsequent action of lipoxygenases (LOXs) on these phospholipids generates lipid hydroperoxides. The uncontrolled accumulation of these hydroperoxides leads to membrane damage and cell death.
Antioxidant Systems
Cells possess robust antioxidant defense systems to prevent ferroptosis. The most critical is the system Xc–/GSH/GPX4 pathway. The system Xc– transporter, a heterodimer of subunits SLC7A11 and SLC3A2, imports cystine in exchange for glutamate. This cystine is rapidly reduced to cysteine, a building block for glutathione (GSH). Glutathione peroxidase 4 (GPX4), a selenoprotein, uses GSH as a cofactor to reduce lipid hydroperoxides to non-toxic lipid alcohols, effectively halting lipid peroxidation. When the system Xc– is inhibited, GSH synthesis is blocked, rendering GPX4 inactive and leaving the cell vulnerable to ferroptosis. Other, parallel antioxidant systems include the FSP1-CoQ10 axis and the GCH1-BH4 pathway, which also play critical roles in defending against lipid peroxidation.
Targeting Ferroptosis for Immunotherapy
The crosstalk between ferroptosis and the immune system is a fertile area for therapeutic innovation. The induction of ferroptotic cell death can release damage-associated molecular patterns (DAMPs), which act as "eat-me" signals for the immune system, transforming an immune-cold tumor into an immune-hot one.
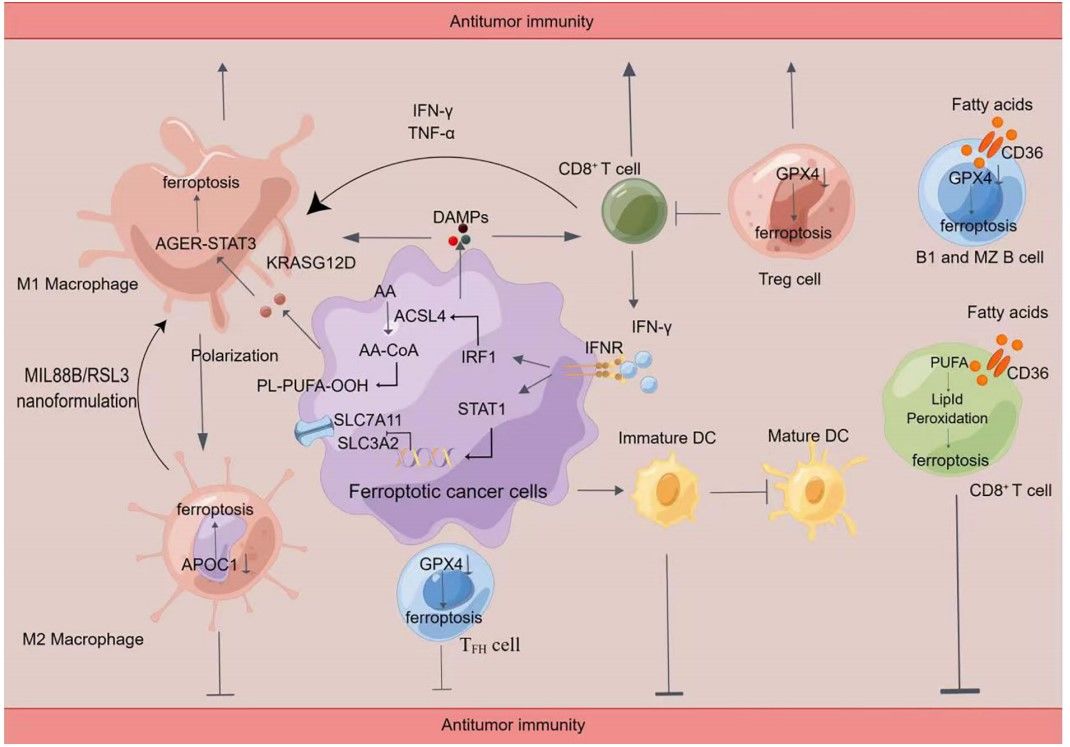 Fig.1 Crosstalk between immune cells and ferroptosis.1
Fig.1 Crosstalk between immune cells and ferroptosis.1
T Cells: Inducing Tumor Cell Death
T cells, particularly cytotoxic CD8+ T cells, are central to the anti-tumor immune response. Recent research has shown that activated CD8+ T cells can induce ferroptosis in tumor cells. When T cells are activated by immunotherapy, they secrete interferon-gamma (IFN-γ). This cytokine activates the JAK1-STAT1 signaling pathway in tumor cells, which subsequently downregulates the expression of the SLC7A11 subunit of the system Xc–. This blocks cystine uptake, depletes GSH, and sensitizes the tumor cells to ferroptosis. This mechanism provides a powerful rationale for combining ferroptosis-inducing agents with T cell-based immunotherapies. However, it is also known that T cells can become susceptible to ferroptosis themselves, such as through CD36-mediated lipid uptake in the tumor microenvironment. Therefore, a dual strategy of promoting ferroptosis in tumor cells while protecting T cells is key.
Macrophages: Reprogramming the Microenvironment
Macrophages are highly plastic immune cells, often polarizing into either pro-tumor M2-like or anti-tumor M1-like phenotypes. M1 macrophages are resistant to ferroptosis due to high levels of nitric oxide synthase (iNOS), which inhibits lipid peroxidation. Conversely, M2 macrophages lack this protection and are more susceptible to ferroptosis. Ferroptotic cancer cells release DAMPs like HMGB1, which promotes the polarization of macrophages toward the anti-tumor M1 phenotype. This provides a crucial therapeutic opportunity: by using nanoparticles or other agents to specifically induce ferroptosis in the pro-tumor M2 macrophages, the immune microenvironment can be "reprogrammed" to a more anti-tumor state.
B Cells and DCs: Orchestrating the Response
B cells and dendritic cells (DCs) are critical components of the adaptive immune response. Similar to T cells, different B cell subsets show varying sensitivities to ferroptosis, which may be related to their lipid metabolism. Furthermore, the release of DAMPs from cancer cells undergoing ferroptosis can lead to the maturation of DCs, which then present tumor antigens to T cells, further activating a robust adaptive immune response. This orchestration of the immune system via ferroptosis highlights its potential as a central hub for therapeutic strategy.
Immune Checkpoint Blockade: A Powerful Synergy
Immune checkpoint inhibitors (ICIs), such as anti-PD-1/PD-L1, have revolutionized cancer therapy, but their efficacy is limited by primary and acquired resistance. Ferroptosis induction is emerging as a powerful strategy to overcome this resistance. Studies have demonstrated that inhibiting the ferroptosis pathway, for example, by upregulating SLC7A11, is a key resistance mechanism for tumors. By combining ICIs with ferroptosis sensitizers, the anti-tumor immune response can be significantly amplified, turning ICI-resistant tumors into responsive ones and creating a positive feedback loop of tumor elimination.
The therapeutic potential of targeting ferroptosis is immense, offering a new avenue to overcome the limitations of current cancer treatments. Contact Creative Biolabs today to learn how our ferroptosis-related antibody products can accelerate your therapeutic development.
Reference
- Cai, Huazhong et al. "Ferroptosis and tumor immunotherapy: A promising combination therapy for tumors." Frontiers in oncology vol. 13 1119369. 8 Feb. 2023, https://doi.org/10.3389/fonc.2023.1119369. Distributed under Open Access license CC BY 4.0, without modification.


