DCK Antibodies
Background
DCK is a key phosphorylase, mainly present in the cytoplasm of eukaryotic cells. This enzyme participates in the remedial pathways of DNA synthesis and repair by catalyzing the phosphorylation reactions of nucleoside analogues such as deoxycytidine and is crucial for maintaining genomic stability and cell proliferation. DCK holds special value in antiviral and anti-tumor treatments as it can activate various nucleoside drug precursors (such as gemcitabine), thereby enhancing the efficacy of chemotherapy. Scientists first identified its function in the 1970s and subsequently analyzed its three-dimensional structure through X-ray crystallography, revealing the molecular mechanism of substrate recognition. As a core target of drug metabolism, DCK's highly efficient specific binding ability provides an important template for the design of new anti-cancer drugs and promotes the development of the field of precision medicine.
Structure of DCK
DCK is a key phosphorylase with a molecular weight of approximately 30.5 kDa. Its size varies slightly among different species, mainly depending on minor changes in the amino acid sequence.
| Species | Human | Mice | Rats | Bovine |
| Molecular Weight (kDa) | 30.5 | 30.3 | 30.4 | 30.6 |
| Primary Structural Differences | Conserved catalytic core with consistent key active sites | Highly homologous to human DCK (>85%) | There are individual variations in the substrate binding region | Slightly lower enzyme activity but still similar functions |
DCK is usually composed of around 260 amino acids and presents a typical α/β folding structure. Its tertiary structure contains a nucleotide-binding pocket, composed of multiple β -sheets and α -helixes, which can specifically recognize substrates such as deoxycytidine. Key aspartic acid residues (such as D86) are involved in the phosphate transfer reaction, while the magnesium ion binding site (usually coordinated by D132) is crucial for catalytic activity. The active center of this enzyme is highly conserved, ensuring its efficiency and selectivity in nucleotide metabolism.
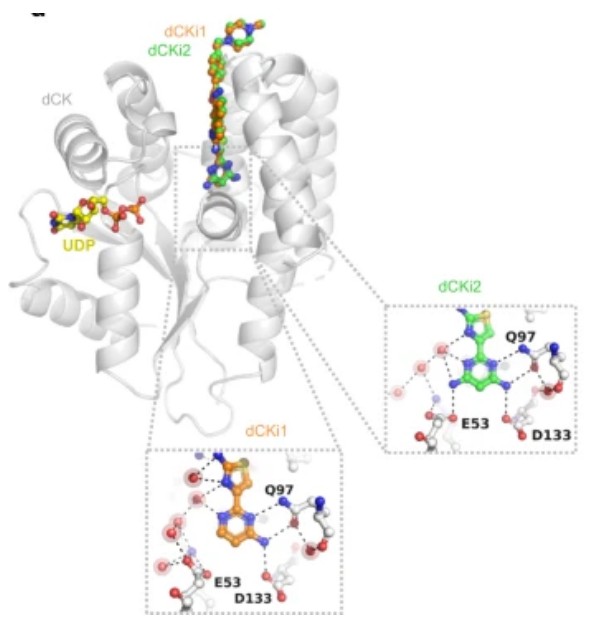 Fig. 1 Superimposition of crystal structures of dCK (gray ribbons) in complex with UDP (yellow) and dCKi1 (orange).1
Fig. 1 Superimposition of crystal structures of dCK (gray ribbons) in complex with UDP (yellow) and dCKi1 (orange).1
Key structural properties of myoglobin:
- α/β folding structure
- Conservative catalytic pocket
- Magnesium ion-dependent phosphate transfer center
- Dynamic active regulation loop
Functions of DCK
The core function of DCK (deoxycytidine kinase) is to catalyze the nucleotide salvage synthesis pathway, and it also plays a key role in anti-tumor treatment. Its specific functions are as follows:
| Function | Description |
| Nucleotide phosphorylation | Phosphorylating nucleoside analogues such as deoxycytidine to monophosphate form initiates the remedial synthesis pathway of DNA synthesis raw materials. |
| Activation of chemotherapy drugs | Specifically phosphorylate the precursors of anti-cancer drugs such as gemcitabine and cytarabine, converting them into active therapeutic forms. |
| DNA damage repair | By maintaining the balance of the dNTP library, it supports the normal operation of the DNA damage repair system. |
| Cell cycle regulation | During the S phase, the activity is significantly increased, ensuring the supply of nucleotides necessary for DNA replication. |
| Virus replication inhibition | Phosphorylated antiviral nucleoside analogues (such as zidovudine) block the function of viral polymerase. |
The enzymatic kinetic characteristics of DCK are manifested as a typical Mie equation curve, and its catalytic efficiency is strictly regulated by the concentration of the ATP-Mg²⁺ complex. The Km value of this enzyme for natural substrates (deoxycytidyl) is approximately 1-5μM, while for chemotherapy drugs (such as gemcitabine), it is higher (10-20μM). This difference is used to optimize the design of anti-cancer drugs.
Applications of DCK and DCK Antibody in Literature
1. Song, Danjun, et al. "DCK is a promising prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma." World Journal of Surgical Oncology 18.1 (2020): 176. https://doi.org/10.1186/s12957-020-01953-1
The article shows that DCK is highly expressed in hepatocellular carcinoma (HCC), which is associated with poor prognosis of patients and tumor-infiltrating immune cells (such as Tregs and exhausted T cells), suggesting that it may promote immunosuppression and escape, and can be used as a potential prognostic marker and indicator of immune infiltration status in HCC.
2. Hu, Qiangsheng, et al. "dCK negatively regulates the NRF 2/ARE axis and ROS production in pancreatic cancer." Cell proliferation 51.4 (2018): e12456. https://doi.org/10.1111/cpr.12456
Research has found that the down-regulation of DCK in pancreatic cancer enhances gemcitabine resistance by inhibiting the NRF2 antioxidant pathway and promoting the accumulation of ROS. Low expression of DCK is associated with elevated NRF2 activity, affecting tumor proliferation and metastasis, revealing the key role of the DCK-NrF2 axis in chemotherapy resistance.
3. Kurata, Morito, et al. "Using genome-wide CRISPR library screening with library resistant DCK to find new sources of Ara-C drug resistance in AML." Scientific reports 6.1 (2016): 36199. https://doi.org/10.1038/srep36199
The article found through CRISPR screening that the absence of DCK in AML led to resistance to cytarabine (Ara-C), but increased sensitivity to prednisolone. Cells deficient in DCK or SLC29A are more sensitive to prednisolone, suggesting that it may be used as an adjuvant treatment strategy for DCK-negative AML.
4. Zhao, Ya-Hui, et al. "DCK confers sensitivity of DCTD-positive cancer cells to oxidized methylcytidines." Protein & Cell 14.7 (2023): 534-539. https://doi.org/10.1093/procel/pwac028
Research has found that oxidative methylcytidine (5hmdC/5fdC) can still induce DNA damage and apoptosis through the DCK-dependent pathway in leukemia cells lacking cytidine deaminase (CDA), suggesting that it can serve as a novel anti-cancer strategy to overcome resistance to traditional cytidine analogues.
5. Buocikova, Verona, et al. "Decitabine-induced DNA methylation-mediated transcriptomic reprogramming in human breast cancer cell lines; the impact of DCK overexpression." Frontiers in pharmacology 13 (2022): 991751. https://doi.org/10.3389/fphar.2022.991751
Research has found that overexpression of DCK in breast cancer cells does not significantly enhance the DNA demethylation or cytotoxicity of decitabine (DAC), but low-dose DAC can induce genome-wide methylation reprogramming and simultaneously activate tumor suppressor genes and oncogenes, suggesting that its efficacy and risks coexist.
Creative Biolabs: DCK Antibodies for Research
Creative Biolabs specializes in the production of high-quality DCK antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom DCK Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our DCK antibodies, custom preparations, or technical support, contact us at email.
Reference
- Saez-Ayala, Magali, et al. "From a drug repositioning to a structure-based drug design approach to tackle acute lymphoblastic leukemia." Nature communications 14.1 (2023): 3079.https://doi.org/10.1038/s41467-023-38668-2
Anti-DCK antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7697) (CBMAB-1869CQ)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-ATP1B1 Recombinant Antibody (E4) (CBMAB-0463-LY)

-
Mouse Anti-DLG1 Monolconal Antibody (4F3) (CBMAB-0225-CN)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-CD33 Recombinant Antibody (P67.6) (CBMAB-C10189-LY)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Rat Anti-AChR Recombinant Antibody (V2-12500) (CBMAB-0990-CN)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-ATP1B3 Recombinant Antibody (1E9) (CBMAB-A4021-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-180650) (CBMAB-A2186-YC)

-
Mouse Anti-CD2AP Recombinant Antibody (BR083) (CBMAB-BR083LY)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503417) (CBMAB-V208-1369-FY)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






