IL4 Antibodies
Background
IL4 (interleukin-4) is a small molecule glycoprotein mainly secreted by activated T cells and mast cells, belonging to the cytokine family. It plays a core role in immune regulation, promoting B cell proliferation and antibody class conversion, while regulating Th2-type immune responses, and is crucial for allergic reactions and anti-parasitic defense. IL4 was first identified by William Paul's team in 1982. The crystal structure analysis of its receptor complex (IL4Rα/γc) provided a paradigm for the study of immune signal transduction. This molecule achieves precise receptor recognition through its unique helical beam structure. The research on its functional mechanism has greatly advanced the development process of autoimmune diseases and tumor immunotherapy. In 2017, dupilumab designed based on the IL4 pathway became the first biological agent approved for the treatment of atopic dermatitis.
Structure of IL4
IL4 is a glycoprotein with a molecular weight of approximately 15-20 kDa. Its precise molecular weight varies slightly due to the degree of glycosylation and species differences.
| Species | Human | Mice | Rats |
| Molecular Weight (kDa) | 15-18 | 14-16 | 15-17 |
| Primary Structural Differences | Contains 4 alpha helix, three disulfide bond | Approximately 50% homology to human IL4 | Highly similar to human IL4 |
IL4 is composed of 129 amino acids and features A typical four-helix bundle structure (A-D helix), maintaining stability through three conserved disulfide bonds. Its functionality depends on the binding to the IL4 receptor (IL4Rα/γc), where key sites such as Arg88 and Tyr124 are directly involved in receptor interactions. The secondary structure of IL4 is dominated by α -helices, forming a hydrophobic core, while the surface-exposed charged residues regulate its specific recognition with receptors. This protein plays a core role in immune regulation, particularly influencing the differentiation of Th2 cells and the conversion of IgE categories.
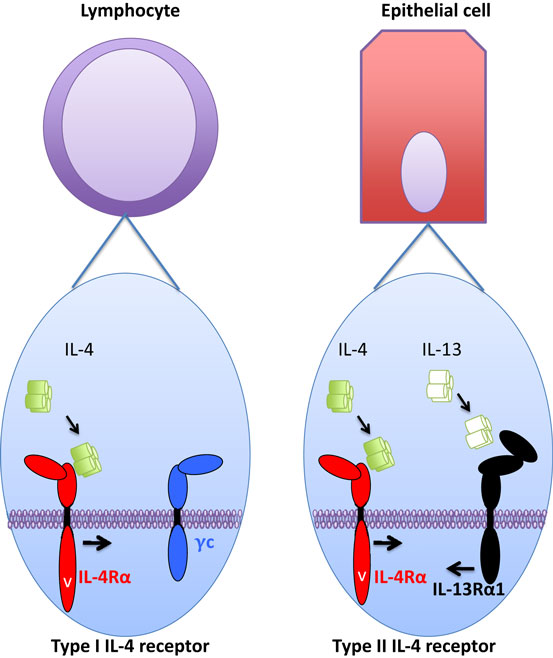 Fig. 1 IL-4 receptor type I/II in hematopoiesis and hematopoietic cell difference expression and functional regulation.1
Fig. 1 IL-4 receptor type I/II in hematopoiesis and hematopoietic cell difference expression and functional regulation.1
Key structural properties of IL4:
- Four-helix bundle structure (A-D helix)
- Conserved disulfide bonds (Cys3-Cys127, Cys24-Cys65, Cys46-Cys99)
- Key receptor binding sites (Arg88, Tyr124, Glu9)
- Glycosylation modification sites (such as Asn38, etc)
- Hydrophobic core and hydrophilic surface
Functions of IL4
The core function of IL4 (interleukin-4) is to regulate immune responses, especially playing a key role in Th2-type immune responses, and it is also involved in various pathophysiological processes.
| Function | Description |
| Th2 cell differentiation | STAT6 signaling pathway can induce naive CD4+ T cells to differentiate into Th2 cells and promote IL-4/IL-5/IL-13 secretion. |
| B-cell category conversion | Induce the conversion of B-cell antibody categories into IgE and IgG1, mediate allergic reactions and anti-parasitic immunity. |
| Macrophage polarization | Drive the activation of M2-type macrophages and participate in tissue repair and fibrosis processes. |
| Inflammatory regulation | Inhibit the production of pro-inflammatory factors (such as TNF-α and IL-1β), and balance the Th1/Th2 immune response. |
| Regulation of the tumor microenvironment | Promote the activation of tumor-associated fibroblasts (CAFs), and influence tumor immune escape and microenvironment remodeling. |
The signal transduction of IL4 shows a biphase concentration dependence: at low concentrations, the STAT6 pathway is preferentially activated, while at high concentrations, the IRS pathway can be additionally activated. This characteristic enables it to precisely regulate the intensity and duration of immune responses. Unlike the broad-spectrum effects of most cytokines, the function of IL4 has significant cell type specificity, such as differentiated regulation of B cells and T cells, highlighting its pivotal position in the immune network.
Applications of IL4 and IL4 Antibody in Literature
1. Junttila, Ilkka S. "Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes." Frontiers in immunology 9 (2018): 888.https://doi.org/10.3389/fimmu.2018.00888
Studies have shown that IL-4 and IL-13 are key cytokines regulating allergic inflammation, which can induce Th2 cell differentiation, IgE category conversion and alternative activation of macrophages. The receptor binding kinetics and chain expression determine the signal efficiency, and the related mechanism research lays the foundation for targeted therapy of allergic diseases. The pioneering work of William E. Paul and others has promoted the development of this field.
2. Zakaria, Marwa, et al. "Role of interleukin 4 (IL4) and interleukin 6 (IL6) in the pathogenesis and prognosis of childhood primary immune thrombocytopenia." European journal of pediatrics 182.7 (2023): 3129-3138. https://doi.org/10.1007/s00431-023-04945-x
Studies have shown that the levels of serum IL-4 and IL-6 in children with immune thrombocytopenia (ITP) are significantly elevated, especially in the initial and persistent ITP. Those with a higher level of IL-4 are more likely to achieve remission, suggesting that it may be involved in the pathogenesis of ITP and can be used as a predictor of treatment response.
3. Steen-Louws, Cristine, et al. "Sialic acid-engineered IL4–10 fusion protein is bioactive and rapidly cleared from the circulation." Pharmaceutical Research 37.2 (2020): 17. https://doi.org/10.1007/s11095-019-2744-y
Research has found that the in vitro and in vivo activities of the IL4-10 fusion protein (IL4-10 FP) with low sialacidification and high sialacidification are similar, but the initial clearance rate of the low sialacidification version is higher, with a half-life of 20.7 minutes for both. Differential sialacidification does not affect the efficacy of the drug, but it can regulate the systemic exposure level. The low sialacidification type is more suitable for local administration to reduce systemic exposure.
4. Piloto, Ana Margarida, et al. "Plastic antibodies tailored on quantum dots for an optical detection of myoglobin down to the femtomolar range." Scientific reports 8.1 (2018): 4944. https://doi.org/10.1155/2013/263952
This study made the first attempt to predict IL4-induced peptides. By analyzing 904 IL4-induced peptides and 742 non-induced peptides, it was found that there were differences in their amino acid composition and motif patterns. The classification model developed based on this has an accuracy rate of 75.76% and can be used to design peptides that induce Th2 immune responses.
5. Zhou, Xiaolai, Björn Spittau, and Kerstin Krieglstein. "TGFβ signalling plays an important role in IL4-induced alternative activation of microglia." Journal of neuroinflammation 9.1 (2012): 210. https://doi.org/10.1186/1742-2094-9-210
Research has found that TGFβ can enhance IL4-induced alternative activation of microglia (M2 type), significantly increasing the expression of Arg1 and Ym1. IL4 can also promote the secretion of TGFβ2, and blocking TGFβ receptor I inhibits this process, indicating that IL4-induced M2 polarization depends on the TGFβ signaling pathway.
Creative Biolabs: IL4 Antibodies for Research
Creative Biolabs specializes in the production of high-quality IL4 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom IL4 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our IL4 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Junttila, Ilkka S. "Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes." Frontiers in immunology 9 (2018): 888.https://doi.org/10.3389/fimmu.2018.00888
Anti-IL4 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-ADV Recombinant Antibody (V2-503423) (CBMAB-V208-1364-FY)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Mouse Anti-COL1A2 Recombinant Antibody (CF108) (V2LY-1206-LY626)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-NSUN6 Recombinant Antibody (D-5) (CBMAB-N3674-WJ)

-
Mouse Anti-ACKR3 Recombinant Antibody (V2-261265) (CBMAB-C1023-LY)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-AMACR Recombinant Antibody (CB34A) (CBMAB-CA034LY)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-ADGRE5 Recombinant Antibody (V2-360335) (CBMAB-C2088-CQ)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-CCN2 Recombinant Antibody (CBFYC-2383) (CBMAB-C2456-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








