ACY1 Antibodies
Background
ACY1 is a cytoplasmic enzyme mainly distributed in the liver and kidneys, belonging to the hydrolase family in the amino acid metabolism system. This enzyme participates in regulating the acetyl metabolism and amino acid cycle balance of the body by specifically hydrolyzing N-acetyl amino acid substrates, and plays a key role in maintaining the homeostasis of the intracellular environment. Since its discovery in the 1970s, researchers have analyzed its three-dimensional structure through X-ray crystal diffraction technology, revealing the unique dual-domain folding mode of the enzyme and the catalytic mechanism of its active center. As a core component of the amino acid metabolic pathway, the functional defect of ACY1 has been confirmed to be associated with a variety of genetic metabolic diseases, and the research on its molecular mechanism provides an important target for the diagnosis and treatment of related diseases.
Structure of ACY1
ACY1 is a hydrolase with a molecular weight of approximately 45.8 kDa. Its precise molecular weight varies slightly among different species, mainly due to minor changes in amino acid sequences.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 45.8 | 45.6 | 45.7 | 45.9 |
| Primary Structural Differences | Conservative catalytic domain | The sequence similarity is high | There is a substitute residue | High homology with humans |
This protein is composed of approximately 408 amino acids and presents a typical bidomain spherical structure. Its primary structure folds to form two main domains: an α/β hydrolase domain at the N-terminal and a β-sandwich domain at the C-terminal, which together constitute the active center. The active center contains a conserved divalent metal ion binding site (usually a zinc ion), which is crucial for catalyzing the hydrolysis reaction of N-acetylated amino acids. Its secondary structure is mainly composed of α -helices and β -folds, which enclose to form a hydrophobic channel for substrate recognition and binding. A highly conserved aspartic acid residue serves as a key catalytic residue, directly participating in the deacetylation process of the substrate.
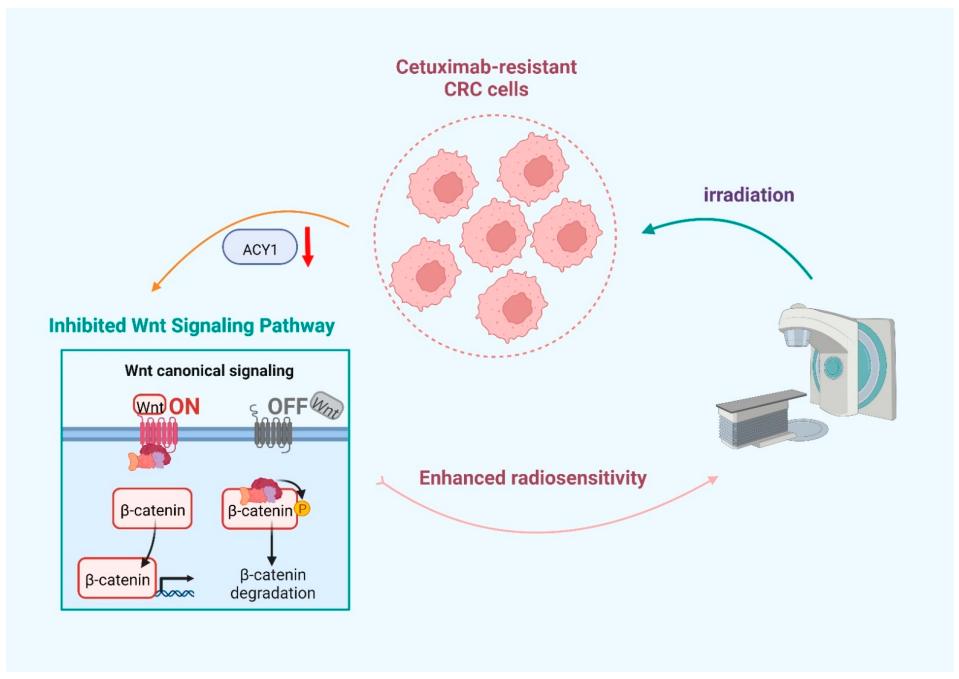 Fig. 1 Schematic of ACY1 downregulation enhances the radiosensitivity of cetuximab-resistant colorectal cancer by inactivating the Wnt/β-catenin signaling pathway.1
Fig. 1 Schematic of ACY1 downregulation enhances the radiosensitivity of cetuximab-resistant colorectal cancer by inactivating the Wnt/β-catenin signaling pathway.1
Key structural properties of ACY1:
- Conservative dual-domain structure (α/β hydrolase domain and β -sandwich domain)
- Active center zinc ion binding sites
- Hydrophobic channels are responsible for substrate identification and binding
Functions of ACY1
The main function of the ACY1 gene is to catalyze the hydrolysis of N-acetylated amino acids and participate in the metabolism and recycling of amino acids within cells. In addition, it also involves a variety of physiological and pathological processes, including the regulation of neural transmission and the occurrence of metabolic diseases.
| Function | Description |
| Deacetylation catalysis | Specifically hydrolyze N-acetylamino acids to generate free amino acids and acetic acid, maintaining the balance of amino acid metabolism. |
| Amino acid recovery | Promote the breakdown and reuse of acetylated amino acids in the kidney and liver, supporting nitrogen balance in the body. |
| Potential role in neuromodulation | May be affected by acetylated amino acid level indirect regulating neurotransmitter synthesis and function. |
| Association with metabolic diseases | ACY1 dysfunction is associated with a variety of hereditary metabolic diseases, such as aminoaciduria and neurological abnormalities. |
| Detoxification auxiliary function | Participate in the elimination of excessive acetylated metabolites in the body and reduce the metabolic pressure on cells. |
This enzyme follows the classic mechanism of metallohydrolase, and its catalytic efficiency depends on the zinc ions in the active center. It shows the highest activity under physiological pH conditions, indicating that it plays an important role in maintaining intracellular homeostasis.
Applications of ACY1 and ACY1 Antibody in Literature
1. Shan, Wulin, et al. "ACY1 Downregulation Enhances the Radiosensitivity of Cetuximab-Resistant Colorectal Cancer by Inactivating the Wnt/β-Catenin Signaling Pathway." Cancers 14.22 (2022): 5704. https://doi.org/10.3390/cancers14225704
This study explores the role of ACY1 in radiotherapy for cetuxi-resistant colorectal cancer. Research has found that after radiotherapy, the expression of ACY1 is down-regulated and enhances the sensitivity of cancer cells to radiotherapy by inhibiting the Wnt/β-catenin signaling pathway. It indicates that targeting ACY1 can become a potential therapeutic strategy for cetuxi-resistant colorectal cancer.
2. Mauri, Alessia, et al. "Menkes disease complicated by concurrent ACY1 deficiency: A case report." Frontiers in Genetics 14 (2023): 1077625. https://doi.org/10.3389/fgene.2023.1077625
This article reports a case of an infant with both Menkes disease and amino acid acylase 1 deficiency, who carried a novel ATP7A mutation and a homozygous variation of c.1057C>T in the ACY1 gene. The clinical manifestations of the two rare diseases are similar but the treatments are different. The ACY1 variant leads to specific N-acetylaminoaciduria.
3. Liu, Baowen, et al. "A novel co-target of ACY1 governing plasma membrane translocation of SphK1 contributes to inflammatory and neuropathic pain." Iscience 26.6 (2023). https://doi.org/10.1016/j.isci.2023.106989
This study found through the Nav1.8 knockout model that the expression of ACY1 in the spinal cord was upregulated in both inflammatory and neuropathic pain. Overexpression of ACY1 can induce pain, while inhibition of ACY1 can relieve pain. Mechanistically, ACY1 activates astrocytes and glutamatergic neurons by promoting the membrane translocation of sphingosine kinase 1. The results indicated that ACY1 was a co-effector protein downstream of Nav1.8 and could serve as a potential new target for the treatment of chronic pain.
4. Posavi, Marijan, et al. "Characterization of Parkinson's disease using blood-based biomarkers: A multicohort proteomic analysis." PLoS medicine 16.10 (2019): e1002931. https://doi.org/10.1371/journal.pmed.1002931
This study, through blood proteomics analysis, found that the expression of amino acid acylase-1 (ACY1) was significantly altered in the plasma of patients with Parkinson's disease (PD), and this was verified in an independent cohort. Low levels of ACY1 are significantly associated with cognitive decline and the risk of progression to mild cognitive impairment or dementia in PD patients, suggesting that ACY1 can serve as a potential blood biomarker for the disease progression of PD.
5. Qi, Sai, et al. "Identification and Validation of Novel Serum Autoantibodies Biomarkers for Staging Liver Fibrosis in Patients With Chronic Hepatitis B." Frontiers in Medicine 8 (2022): 807087. https://doi.org/10.3389/fmed.2021.807087
This study, through protein chip and ELISA verification, found that the autoantibody ACY1 has diagnostic value in the staging of chronic hepatitis B liver fibrosis. Its differential sensitivity for S4 stage liver fibrosis is 74.42%, and its specificity is 60.87%. Moreover, it is not related to age, gender or inflammation level. It is suggested that ACY1 can serve as a potential serum marker for the staging of liver fibrosis.
Creative Biolabs: ACY1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality ACY1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ACY1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ACY1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Shan, Wulin, et al. "ACY1 Downregulation Enhances the Radiosensitivity of Cetuximab-Resistant Colorectal Cancer by Inactivating the Wnt/β-Catenin Signaling Pathway." Cancers 14.22 (2022): 5704. https://doi.org/10.3390/cancers14225704
Anti-ACY1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Mouse Anti-CD24 Recombinant Antibody (SN3) (CBMAB-C1037-CQ)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-FOXL1 Recombinant Antibody (CBXF-0845) (CBMAB-F0462-CQ)

-
Mouse Anti-DLL4 Recombinant Antibody (D1090) (CBMAB-D1090-YC)

-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-CD2AP Recombinant Antibody (BR083) (CBMAB-BR083LY)

-
Mouse Anti-BMI1 Recombinant Antibody (CBYC-P026) (CBMAB-P0108-YC)

-
Rabbit Anti-CCN1 Recombinant Antibody (CBWJC-3580) (CBMAB-C4816WJ)

-
Rabbit Anti-AP2M1 (Phosphorylated T156) Recombinant Antibody (D4F3) (PTM-CBMAB-0610LY)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






