MUC1 Antibodies
Background
Mucin 1 encoded by the MUC1 gene is a high-molecular-weight transmembrane glycoprotein, mainly expressed on the surface of various epithelial cells. This protein builds a physically protective mucus barrier on the cell surface by forming a repetitive sequence domain rich in threonine-serine and undergoing high glycosylation, while also participating in intercellular signal transduction and immune regulation processes. Under physiological conditions, MUC1 protects epithelial tissues from pathogen invasion through its extracellular domain, while its intracellular domain is involved in regulating cell proliferation and differentiation. This gene was first numerically identified by Gendler's research team in 1987 and is the first fully characterized member of the human mucin family. The polymorphic characteristics caused by its unique variable number of tandem repeats and the significant role of abnormal glycosylation in tumorigenesis and development have made it an important research object in the fields of cancer immunotherapy and liquid biopsy, greatly promoting the progress of cross-disciplinary research between glycobiology and tumor immunology.
Structure of MUC1
MUC1 is a high-molecular-weight transmembrane glycoprotein, and its molecular weight varies significantly within the range of approximately 120 to 225 kDa. This fluctuation mainly stems from the polymorphic expression of variable number tandem repeat sequences (VNTRS) in the gene coding region and the varying degrees of glycosylation modification.
| Species | Human | Mouse | Bovine | Rhesus monkey |
| Molecular Weight (kDa) | 120-225 | 110-180 | 130-210 | 125-220 |
| Primary Structural Differences | Contains 20 to 125 VNTR domains | The repetitive unit of VNTR is relatively short | The glycosylation pattern shows species specificity | With the highly homologous human (MUC1) structure |
This protein is composed of an extracellular domain, a transmembrane region and an intracellular tail. Its extracellular segment is rich in serine/threonine residues, which form an extended sugar chain brush-like structure through O-glycosylation modification, constituting a physical barrier and mediating intercellular communication. The transmembrane region is anchored to the membrane by a single hydrophobic α -helix, while the intracellular tail contains multiple phosphorylation sites that participate in the regulation of cell proliferation and differentiation by recruiting downstream signaling molecules. This unique "one protein, three functions" structural model enables it to play a core role in maintaining epithelial homeostasis and tumorigenesis.
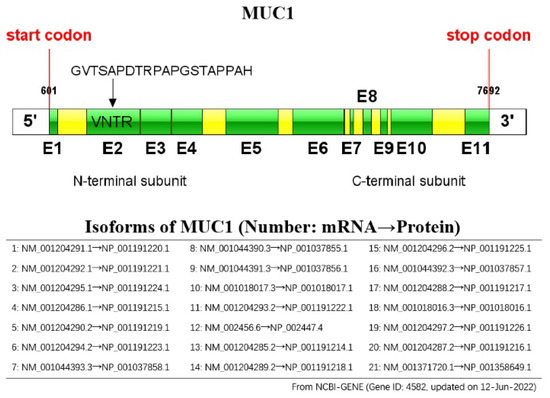 Fig. 1 Schematic representation of the MUC1 gene.1
Fig. 1 Schematic representation of the MUC1 gene.1
Key structural properties of MUC1:
- Highly glycosylated extracellular mucin repeat sequence domains
- Single transmembrane α -helical anchoring region
- Intracellular tail containing multiple die bodies can be phosphorylated signal
Functions of MUC1
The core function of MUC1 is to maintain the integrity of the epithelial barrier and regulate cellular signal transduction. The abnormal expression of this gene is closely related to the occurrence and development of various cancers.
| Function | Description |
| Formation of physical barriers | Extracellular domains form steric hindrance through high glycosylation, protecting epithelial cells from pathogen invasion and enzymatic damage. |
| Cell signal transduction | The intracellular tail is cleaved by proteolysis and enters the nucleus, acting as a co-activator to regulate tumor-related pathways such as Wnt/β-catenin. |
| Immune regulatory effect | Variable number tandem repeat sequences (VNTRS) exhibit individual-specific polymorphisms and affect immune recognition by altering the glycosylation pattern. |
| Promotion of tumor progression | Abnormal glycosylation (such as Tn and sTn antigens) occurs in malignant tumors, leading to the loss of cell adhesion function and promoting metastasis. |
| Development of therapeutic targets | The tumor specific sugar table has become a CAR - T, antibody drug coupling and other important molecular targeted therapy of targets. |
Under normal physiological conditions, MUC1 presents as a transmembrane morphology. However, during the process of malignant transformation, it often undergoes self-splicing and forms stable extracellular segmental - intracellular segment heterodimers. This conformational change leads to its transformation from a protective molecule to a oncogenic signal transducer, demonstrating its dual functionality in maintaining cellular homeostasis and in pathological conditions.
Applications of MUC1 and MUC1 Antibody in Literature
1. Li, Zhifeng, et al. "Advances in MUC1-mediated breast cancer immunotherapy." Biomolecules 12.7 (2022): 952. https://doi.org/10.3390/biom12070952
The article indicates that MUC1 has become an important immunotherapy target due to its overexpression and abnormal glycosylation in breast cancer. This article reviews the research progress and clinical exploration of various breast cancer immunotherapies targeting MUC1, and looks forward to the future development direction.
2. Taylor-Papadimitriou, Joyce, et al. "Latest developments in MUC1 immunotherapy." Biochemical Society Transactions 46.3 (2018): 659-668. https://doi.org/10.1042/BST20170400
The article indicates that although MUC1-based therapies have not yet achieved widespread clinical success, the enthusiasm for their research as cancer immunotherapy targets remains undiminished. This article summarizes the existing attempts of MUC1 immunotherapy and explores how to improve treatment strategies based on new discoveries about its structure and function.
3. Yamashita, Nami, and Donald Kufe. "Addiction of cancer stem cells to MUC1-C in triple-negative breast cancer progression." International Journal of Molecular Sciences 23.15 (2022): 8219. https://doi.org/10.3390/ijms23158219
The article indicates that in triple-negative breast cancer, the MUC1-C protein is a key target driving the self-renewal and malignant progression of tumor stem cells. It promotes tumor drug resistance and immune escape by inducing inflammatory responses and epigenetic remodeling. At present, CAR-T therapy and antibody-drug conjugates targeting MUC1-C have entered the clinical research and development stage, and are expected to provide new strategies for the treatment of this stubborn cancer.
4. Kim, Sojin, et al. "Inhibition of MUC1 exerts cell-cycle arrest and telomerase suppression in glioblastoma cells." Scientific reports 10.1 (2020): 18238. https://doi.org/10.1038/s41598-020-75457-z
The article indicates that in glioblastoma, elevated MUC1 expression is associated with a poor prognosis. Studies have shown that inhibiting MUC1 can suppress tumor proliferation by regulating the cell cycle, reducing telomerase activity and inducing telomere substitution elongation mechanisms. These findings establish the potential of MUC1 as a new therapeutic target for GBM.
5. Shahrad, Shima, et al. "Targeting lung cancer cells with MUC1 aptamer-functionalized PLA-PEG nanocarriers." Scientific Reports 12.1 (2022): 4718. https://doi.org/10.1038/s41598-022-08759-z
In this study, a nano-drug delivery system targeting MUC1 was successfully prepared. Experiments have shown that the anti-cancer drug of this carrier significantly increases the apoptosis induction rate of lung cancer cells with high MUC1 expression, which is 3.2 times that of non-targeted carriers and 8.3 times that of free drugs, respectively, demonstrating excellent potential for targeted therapy.
Creative Biolabs: MUC1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality MUC1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MUC1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MUC1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Li, Zhifeng, et al. "Advances in MUC1-mediated breast cancer immunotherapy." Biomolecules 12.7 (2022): 952. https://doi.org/10.3390/biom12070952
Anti-MUC1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-CAT Recombinant Antibody (724810) (CBMAB-C8431-LY)

-
Mouse Anti-BBS2 Recombinant Antibody (CBYY-0253) (CBMAB-0254-YY)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0435) (CBMAB-0437-YY)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-CIITA Recombinant Antibody (CBLC160-LY) (CBMAB-C10987-LY)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-FOSB Recombinant Antibody (CBXF-3593) (CBMAB-F2522-CQ)

-
Rabbit Anti-ABL1 (Phosphorylated Y185) Recombinant Antibody (V2-443434) (PTM-CBMAB-0001YC)

-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-DHFR Recombinant Antibody (D0821) (CBMAB-D0821-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








