PASK Antibodies
Background
The PASK gene encodes a serine/threonine protein kinase, which serves as a key receptor for cellular metabolic status and senses intracellular energy changes and regulates metabolic balance through its PAS domain. It can regulate key physiological processes such as insulin secretion, glycogen metabolism and mitochondrial biosynthesis based on the availability of nutrients (such as glucose). This gene was identified in the early 21st century. Its unique dual-kinase domain (one for substrate phosphorylation and the other for self-regulation) provides an important model for studying the energy sensing mechanism. The activity regulation mechanism of PASK and its role in metabolic diseases have become an important research direction in the current field of signal transduction and metabolic regulation, providing a molecular basis for understanding how cells coordinate the relationship between energy supply and demand.
Structure of PASK
PASK is a serine/threonine protein kinase with a molecular weight of approximately 150-160 kDa. This protein contains the N-terminal PAS domain and the C-terminal kinase domain, and has highly conserved sequence characteristics in different mammals.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 155 | 153 | 154 | 155 |
| Primary Structural Differences | Contains 1253 amino acids, two-domain architecture | The sequence similarity of PAS domains reaches 95% | Highly conservative kinase domain structure | Structure of domain and human are basically identical |
The PAS domain of the PASK protein is responsible for sensing the cell's energy state, while the kinase domain performs phosphorylation functions. The two domains regulate each other through allosteric mechanisms: after the PAS domain senses changes in metabolites, it induces conformational changes in kinases, thereby regulating their catalytic activity. This unique dual-domain architecture enables PASK to precisely coordinate cellular metabolism and energy balance, playing a core role in glucose sensing and mitochondrial function regulation.
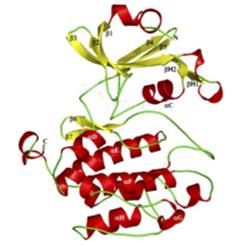 Fig. 1 The crystal structure of the PAS domain of PASK.1
Fig. 1 The crystal structure of the PAS domain of PASK.1
Key structural properties of PASK:
- Unique dual-domain architecture (PAS sensing domain and kinase catalytic domain)
- PAS structure domain as a sensing module of intracellular metabolites
- Kinase domains perform substrate phosphorylation functions
- Allosteric regulatory mechanisms between dual domains control kinase activity
Functions of PASK
The core function of the PASK gene is to serve as a sensor for the energy state of cells. However, it is also widely involved in the regulation of various physiological processes, including metabolic adaptation, autophagy and developmental regulation.
| Function | Description |
| Energy perception | The PAS domain senses the changes in the levels of metabolites such as NADPH and ATP within cells to respond to nutritional availability. |
| Metabolic regulation | Phosphorylates downstream targets and regulates metabolic pathways such as glycogen synthesis, glucose uptake and mitochondrial biosynthesis. |
| Adaptation to hypoxia | Regulating gene expression under hypoxic conditions helps cells adapt to energy stress states and maintain metabolic homeostasis. |
| Autophagy regulation | Through the interaction of pathways such as mTOR, it affects the autophagy process of cells, coordinates energy balance and eliminates damaged components. |
| Developmental support | Regulate cell differentiation and tissue construction at specific stages of embryonic development to ensure energy supply and signal integration during the development process. |
The activity of PASK is strictly controlled by its dual-domain allosteric regulatory mechanism: after the PAS domain senses metabolic signals, it induces conformational changes in the kinase domain, thereby precisely initiating or inhibiting its phosphorylation function. This unique regulatory mode enables PASK to effectively integrate multiple nutritional signals and play a core role in maintaining metabolic homeostasis.
Applications of PASK and PASK Antibody in Literature
1. Zhang, Dan-dan, et al. "Per-Arnt-Sim Kinase (PASK): An emerging regulator of mammalian glucose and lipid metabolism." Nutrients 7.9 (2015): 7437-7450. https://doi.org/10.3390/nu7095347
The article indicates that the nutrition-sensing kinase PASK regulates glycolipid metabolism. Inhibiting PASK can improve obesity and insulin resistance caused by a high-fat diet, making it a potential new therapeutic target for metabolic syndrome.
2. Hurtado-Carneiro, Verónica, et al. "Preventing oxidative stress in the liver: an opportunity for glp-1 and/or pask." Antioxidants 10.12 (2021): 2028. https://doi.org/10.3390/antiox10122028
The article indicates that research has found that inhibiting PASK can improve metabolic abnormalities caused by high-fat diets by regulating signals such as AMPK/mTOR, and work in synergy with GLP-1 to alleviate liver oxidative stress.
3. Xiao, Michael, et al. "PASK links cellular energy metabolism with a mitotic self-renewal network to establish differentiation competence." Elife 12 (2023): e81717. https://doi.org/10.7554/eLife.81717
Research has found that stem cells activate PASK through glutamine metabolism and promote its entry into the nucleus, thereby inhibiting the self-renewal factor Pax7, generating heterogeneity and initiating differentiation, and driving muscle regeneration.
4. Kikani, Chintan K., et al. "Pask integrates hormonal signaling with histone modification via Wdr5 phosphorylation to drive myogenesis." Elife 5 (2016): e17985. https://doi.org/10.7554/eLife.17985
The article indicates that PASK converts the inhibitory histone marker H3K4me1 into the activating H3K4me3 by phosphorylating Wdr5, thereby opening up the expression of differentiation genes (such as sarcoplasmin) and initiating cell differentiation.
5. Pérez-García, Ana, et al. "Storage and utilization of glycogen by mouse liver during adaptation to nutritional changes are GLP-1 and PASK dependent." Nutrients 13.8 (2021): 2552. https://doi.org/10.3390/nu13082552
Research has found that Exendin-4 (a GLP-1 analogue) inhibits PASK by simulating the fasting effect, alters the balance of metabolic enzymes in the liver, and leads to abnormal accumulation of glycogen in the liver even in a fasting state.
Creative Biolabs: PASK Antibodies for Research
Creative Biolabs specializes in the production of high-quality PASK antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom PASK Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our PASK antibodies, custom preparations, or technical support, contact us at email.
Reference
- Zhang, Dan-dan, et al. "Per-Arnt-Sim Kinase (PASK): An emerging regulator of mammalian glucose and lipid metabolism." Nutrients 7.9 (2015): 7437-7450. https://doi.org/10.3390/nu7095347
Anti-PASK antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-2C TCR Recombinant Antibody (V2-1556) (CBMAB-0951-LY)

-
Mouse Anti-ENO1 Recombinant Antibody (CBYC-A950) (CBMAB-A4388-YC)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Mouse Anti-EIF4G1 Recombinant Antibody (2A9) (CBMAB-A2544-LY)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-ARID3A Antibody (A4) (CBMAB-0128-YC)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-CAT Recombinant Antibody (724810) (CBMAB-C8431-LY)

-
Mouse Anti-CCNH Recombinant Antibody (CBFYC-1054) (CBMAB-C1111-FY)

-
Mouse Anti-ATP1A2 Recombinant Antibody (M7-PB-E9) (CBMAB-A4013-YC)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






