SNAP25 Antibodies
Background
SNAP25 is a soluble NSF attachment protein located on the cytoplasmic membrane, mainly distributed at the presynaptic terminals of nerve cells. The protein encoded by this gene mediates the anchoring and fusion process of synaptic vesicles with the cell membrane by forming a SNARE complex with synaptic vesicle membrane proteins and voltage-gated calcium channels, thereby regulating the release efficiency of neurotransmitters. Research has found that the single nucleotide polymorphism of the SNAP25 gene is associated with the risk of developing neuropsychiatric disorders such as attention deficit hyperactivity disorder and schizophrenia, and changes in its expression level directly affect synaptic plasticity. This gene was first identified in 1993. Its unique feature of having no transmembrane domain makes it a classic model for studying the assembly mechanism of the SNARE complex and has made significant contributions to revealing the molecular principles of neuronal exocytosis.
Structure of SNAP25
SNAP25 is a presynaptic membrane protein with a molecular weight of approximately 25 kDa. Its precise molecular weight varies by about 1-2 kDa among different mammals, and this fluctuation mainly results from the structural polymorphism of the cysteine tandem repeat region in the amino acid sequence.
| Species | Human | Mouse | Rat | Cattle | Chicken |
| Molecular Weight (kDa) | 25.0 | 24.8 | 24.9 | 25.1 | 23.7 |
| Primary Structural Differences | Containing 206 amino acids, no across the membrane structure field | Cysteine clusters are highly conserved | The sequence consistency of the SNARE domain reaches 95% | The C-terminal palmitoylation site is retained | Simplified structure of homologous proteins |
This protein is composed of 206 amino acids and spontaneously assemples with syntaxin 1A and VAMP2 to form a quadruhelix bundle through two independent coiled helical domains (SNARE motifs). Its central hydrophobic layer is composed of highly conserved cysteine residues forming cetane acylation modification sites, and dynamic anchoring with the plasma membrane is achieved through palmitoylation modification. The N-terminal and C-terminal SNARE mods adopt parallel and anti-parallel orientations respectively, and drive the fusion process of synaptic vesicles and plasma membranes through a progressive zipper mechanism.
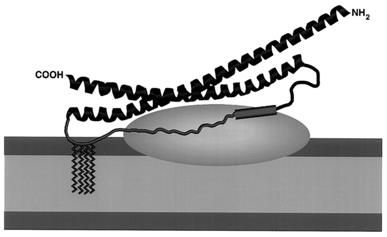 Fig. 1 Model of membrane interactions of SNAP-25.1
Fig. 1 Model of membrane interactions of SNAP-25.1
Key structural properties of SNAP25:
- Soluble membrane proteins without typical transmembrane domains
- SNARE core motif consisting of two coiled helices
- Central cysteine serial sequences mediate palmitoylation modifications
- N-terminal and C-terminal helices are assembled in a parallel - antiparallel mixed orientation
Functions of SNAP25
The core function of SNAP25 is to mediate the fusion process of synaptic vesicles and plasma membranes in neurons. However, it is also involved in the regulation of various neurophysiological activities, including synaptic plasticity modification and the fine regulation of neurotransmitter release dynamics.
| Function | Description |
| Vesicle anchoring | By binding its N-terminal and C-terminal SNARE mods to the plasma membrane protein Syntaxin and the vesicle protein VAMP2 respectively, a trans-SNARE complex is formed, achieving the pre-anchoring of vesicles in the active region. |
| Calcium-triggered fusion | After the calcium influx caused by the action potential, the central hydrophobic layer undergoes conformational rearrangement with Syntaxin-1A, driving the trans-SNARE complex to transform into a cis configuration and catalyzing the formation of membrane fusion pores. |
| Release dynamics regulation | Different splicing isomers of this gene (such as SNAP25a and SNAP25b) precisely regulate the kinetic rate and synchrony of vesicle fusion by altering the degree of palmitoylation modification, thereby influencing short-term plasticity. |
| Synaptic plasticity support | Through its interaction with regulatory factors such as complex proteins and calcium channels, it participates in molecular events that cause persistent changes in synaptic efficacy, such as long-term enhancement, and serves as the molecular basis for learning and memory. |
| Neuroprotection | Maintaining normal neurotransmitter release efficiency is crucial for the homeostasis of neural networks, and its dysfunction is closely related to cognitive deficits and the pathological processes of various neuropsychiatric disorders. |
Unlike the single-site binding mode of Syntaxin-1A, SNAP25 forms a dynamic interaction network with multiple partner proteins through its double SNARE motif, which enables it to integrate various regulatory signals and play a core hub role in synaptic transmission.
Applications of SNAP25 and SNAP25 Antibody in Literature
1. Wang, Wei, et al. "Neuronal-specific TNFAIP1 ablation attenuates postoperative cognitive dysfunction via targeting SNAP25 for K48-linked ubiquitination." Cell Communication and Signaling 21.1 (2023): 356. https://doi.org/10.1186/s12964-023-01390-z
The article indicates that TNFAIP1 promotes postoperative cognitive impairment by mediating the ubiquitination degradation at the K48 position of SNAP25, inhibiting mitochondrial autophagy and exacerbating pyroptosis. Inhibiting TNFAIP1 can protect SNAP25, improve cognition, and may become a new therapeutic strategy.
2. Chen, Zhiqiang, et al. "SNAP25-induced MYC upregulation promotes high-grade neuroendocrine lung carcinoma progression." Frontiers in Immunology 15 (2024): 1411114. https://doi.org/10.3389/fimmu.2024.1411114
Studies have found that SNAP25 is highly expressed in high-grade neuroendocrine carcinomas and promotes c-MYC expression by activating the MEK/ERK pathway, driving tumor progression. Knocking down SNAP25 can inhibit the proliferation and migration of cancer cells, indicating its potential as a therapeutic target.
3. Di, Longjiang, et al. "SNAP25 is a potential prognostic biomarker for prostate cancer." Cancer Cell International 22.1 (2022): 144. https://doi.org/10.1186/s12935-022-02558-2
Studies have revealed that SNAP25 is significantly lowly expressed in prostate cancer and is associated with a poor prognosis. Its expression level is positively correlated with the degree of immune cell infiltration, indicating that SNAP25 is a potential prognostic biomarker.
4. Huang, Qiongzhen, et al. "SNAP25 inhibits glioma progression by regulating synapse plasticity via GLS-mediated Glutaminolysis." Frontiers in Oncology 11 (2021): 698835. https://doi.org/10.3389/fonc.2021.698835
Research reveals that SNAP25 is lowly expressed in glioma and has a poor prognosis. It exerts anti-cancer effects by inhibiting GLS-mediated glutamine metabolism and dendrite formation, and is a potential new therapeutic target.
5. Lin, Ziqi, and Hang Fai Kwok. "RUNDC3A/SNAP25/Akt signaling mediates tumor progression and chemoresistance in gastric neuroendocrine carcinoma." Cell Death & Disease 13.10 (2022): 840. https://doi.org/10.1038/s41419-022-05294-7
Research has revealed that SNAP25 drives tumor progression and chemotherapy resistance in neuroendocrine carcinoma by stabilizing Akt, and its upstream is regulated by RUNDC3A, which is a potential therapeutic target.
Creative Biolabs: SNAP25 Antibodies for Research
Creative Biolabs specializes in the production of high-quality SNAP25 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom SNAP25 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our SNAP25 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Gonzalo, Susana, Wendy K. Greentree, and Maurine E. Linder. "SNAP-25 is targeted to the plasma membrane through a novel membrane-binding domain." Journal of Biological Chemistry 274.30 (1999): 21313-21318.https://doi.org/10.1074/jbc.274.30.21313
Anti-SNAP25 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Rabbit Anti-ALK (Phosphorylated Y1278) Recombinant Antibody (D59G10) (PTM-CBMAB-0035YC)

-
Mouse Anti-ENO1 Recombinant Antibody (8G8) (CBMAB-E1329-FY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-CD247 Recombinant Antibody (6B10.2) (CBMAB-C1583-YY)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Mouse Anti-BACE1 Recombinant Antibody (61-3E7) (CBMAB-1183-CN)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-ACLY Recombinant Antibody (V2-179314) (CBMAB-A0610-YC)

-
Mouse Anti-CEMIP Recombinant Antibody (3C12) (CBMAB-K0296-LY)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








