THRA Antibodies
Background
THRA is a nuclear receptor protein, mainly distributed in tissues such as the heart, skeletal muscle and central nervous system. This protein participates in key physiological processes such as cell metabolism, growth and development, and energy balance by binding to thyroid hormones and regulating the transcriptional expression of target genes. As an important member of the nuclear receptor superfamily, THRA plays an irreplaceable role in embryonic development and the maintenance of adult tissue function. The three-dimensional structure analysis reveals the precise collaboration mechanism between the hormone-binding domain and the DNA-binding domain, providing a classic example for understanding nuclear receptor signal transduction. Mutations in the THRA gene can lead to thyroid hormone resistance syndrome, presenting symptoms such as growth retardation and bradycardia. This discovery has deepened people's understanding of the endocrine regulatory network. The research on this receptor not only promotes the improvement of nuclear receptor theory, but also provides new ideas for targeted therapy of related metabolic diseases.
Structure of THRA
THRA is a nuclear receptor protein with a molecular weight of approximately 48-55 kDa. Its precise molecular weight varies slightly due to different subtypes (such as TRα1 and TRα2) and post-translational modifications.
| Species | Human | Mice | Rats | Zebrafish | Chickens |
| Molecular Weight (kDa) | 48-55 | 48-54 | 49-55 | 50-56 | 47-53 |
| Primary Structural Differences | Containing DNA-binding domains (DBD) and ligand-binding domains (LBD) | Highly homologous to humans | Conservative hormone binding sites | Retain the core functions but have different affinities | Evolutionarily conserved receptor structure |
THRA is composed of approximately 400 amino acids, and its structure includes a DNA binding domain (DBD) and a ligand-binding domain (LBD), forming a typical nuclear receptor folding pattern. DBD binds to the thyroid hormone response element (TRE) of the target gene through the zinc finger structure, while LBD is responsible for recognizing and binding to thyroid hormones (T3/T4). After the receptor is activated, its conformational changes promote the recruitment of co-regulatory factors, which in turn regulate the transcription of downstream genes. The ligand binding pocket of THRA is composed of hydrophobic amino acids, among which key residues (such as Arg228 and His381) are involved in hormone binding and signal transduction. The abnormal function of this receptor is closely related to metabolic disorders, heart diseases and developmental defects.
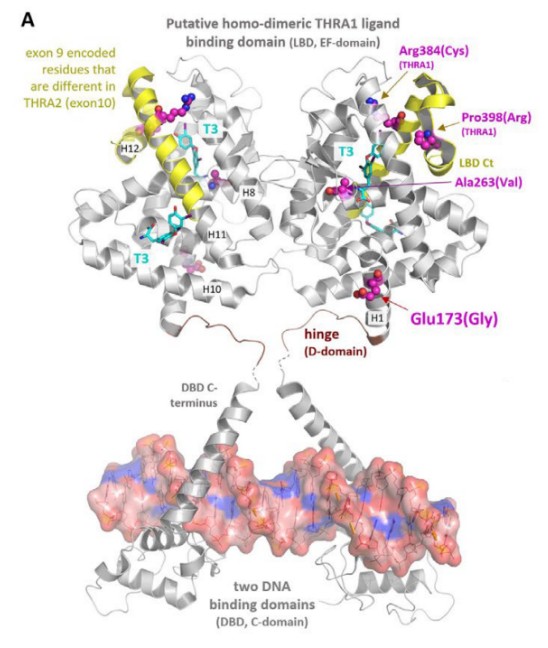 Fig. 1 Structural modeling of THRA ligandbinding domain dimer reveals the site of action of p.(E173G) variant.1
Fig. 1 Structural modeling of THRA ligandbinding domain dimer reveals the site of action of p.(E173G) variant.1
Key structural properties of myoglobin:
- Modular domain composition
- Zinc finger structure mediates DNA recognition
- Hydrophobic ligand-binding pocket
- Key functional residues
- Dimerization ability
Functions of THRA
The main function of THRA is to mediate the genomic effects of thyroid hormones, and it also plays an important role in non-genomic regulation.
| Function | Description |
| Regulation of gene expression | As a ligand-dependent transcription factor, THRA, after binding to thyroid hormones (T3/T4), regulates their transcriptional activity by binding to the thyroid hormone response element (TRE) of the target gene, thereby influencing cell metabolism, proliferation and differentiation. |
| Developmental regulation | During embryonic development, THRA is crucial for the normal development of the central nervous system, skeletal system and cardiovascular system. Knockout animal models have shown that its defects can lead to growth retardation and intellectual disability. |
| Metabolic regulation | THRA plays a core role in maintaining basal metabolic rate and energy balance by regulating mitochondrial biosynthesis, glucose metabolism and lipid catabolism. |
| Regulation of cardiac function | In cardiomyocytes, THRA regulates the expression of ion channels and contractile proteins, influencing heart rate and myocardial contractility. Its abnormalities are associated with arrhythmia and heart failure. |
| Regulation of non-genomic signals | In addition to classical transcriptional regulation, THRA can rapidly regulate cellular stress responses and proliferation by interacting with signaling pathways such as PI3K and MAPK. |
The hormone-binding kinetics of THRA shows high affinity (Kd≈10^-10 M), enabling it to sensitively respond to changes in circulating thyroid hormone levels. Compared with THRB of the same family, THRA dominates in certain tissues (such as the heart and skeletal muscle), and its abnormal function can lead to various diseases such as thyroid hormone resistance syndrome, metabolic disorders and developmental defects.
Applications of THRA and THRA Antibody in Literature
1. Giolito, Maria Virginia, et al. "Regulation of the THRA gene, encoding the thyroid hormone nuclear receptor TRα1, in intestinal lesions." Molecular Oncology 16.22 (2022): 3975-3993. https://doi.org/10.1002/1878-0261.13298
Studies have confirmed that the THRA gene (encoding the thyroid hormone receptor TRα1) is highly expressed at the bottom of the intestinal crypt, overlapped with the Wnt/Notch signaling, and is upregulated in colorectal cancer. Studies have found that TCF7L2 (Wnt) and CDX2 activate THRA transcription, while RBPJ (Notch) inhibits its expression, revealing the role of the WNT-TR α1 bidirectional regulatory circuit in intestinal epithelial homeostasis and tumorigenesis.
2. Zhang, Chengcheng, Yiwei He, and Lu Liu. "Identifying therapeutic target genes for migraine by systematic druggable genome-wide Mendelian randomization." The Journal of Headache and Pain 25.1 (2024): 100.https://doi.org/10.1186/s10194-024-01805-3
Research has confirmed that based on Mendelian randomization analysis, 21 drugable genes, including THRA, are significantly associated with migraine. Among them, HMGCR and TGFB3 play key roles in both blood and brain tissue, providing a new direction for targeted therapy.
3. Graffunder, Adina Sophie, et al. "Spatiotemporal expression of thyroid hormone transporter MCT8 and THRA mRNA in human cerebral organoids recapitulating first trimester cortex development." Scientific Reports 14.1 (2024): 9355. https://doi.org/10.1038/s41598-024-59533-2
Research has found that in the development of human brain organoids, the THRA gene is expressed in the early stage of the neuroepithelium and is significantly enhanced in excitatory neurons. Combined with the upregulation of the T3 response gene, it is confirmed that this model can be used to study the role of thyroid hormones in cortical neurogenesis.
4. Hess, Julian, et al. "Rapid diagnostic of Streptococcus suis in necropsy samples of pigs by thrA-based loop-mediated isothermal amplification assay." Microorganisms 11.10 (2023): 2447. https://doi.org/10.3390/microorganisms11102447
The research developed a novel LAMP detection method based on the thrA gene. Combined with the LPTV boiling method, it can rapidly detect Streptococcus suis in porcine brain swabs, with a sensitivity of 88% and a specificity of 95.8%, providing an efficient tool for clinical diagnosis.
5. Paisdzior, Sarah, et al. "A new mechanism in THRA resistance: the first disease-associated variant leading to an increased inhibitory function of THRA2." International Journal of Molecular Sciences 22.10 (2021): 5338.https://doi.org/10.3390/ijms22105338
Research has found that a novel missense mutation of the THRA gene (p.E173G) leads to functional acquisition changes, abnormally enhanced transcriptional activity of THRA1, and intensified antagonistic effect of THRA2, revealing for the first time the mechanism by which dual functional abnormalities of THRA cause thyroid hormone disorder symptoms.
Creative Biolabs: THRA Antibodies for Research
Creative Biolabs specializes in the production of high-quality THRA antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom THRA Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our THRA antibodies, custom preparations, or technical support, contact us at email.
Reference
- Paisdzior, Sarah, et al. "A new mechanism in THRA resistance: the first disease-associated variant leading to an increased inhibitory function of THRA2." International Journal of Molecular Sciences 22.10 (2021): 5338.https://doi.org/10.3390/ijms22105338
Anti-THRA antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-BIRC5 Recombinant Antibody (6E4) (CBMAB-CP2646-LY)

-
Mouse Anti-HTLV-1 gp46 Recombinant Antibody (CBMW-H1006) (CBMAB-V208-1154-FY)

-
Mouse Anti-FLT1 Recombinant Antibody (11) (CBMAB-V0154-LY)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Rabbit Anti-CCN1 Recombinant Antibody (CBWJC-3580) (CBMAB-C4816WJ)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Mouse Anti-ATP1B1 Recombinant Antibody (E4) (CBMAB-0463-LY)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








