ASPH Antibodies
Background
The ASPH gene encodes aspartic acid β-hydroxylase, a type II transmembrane protein located in the endoplasmic reticulum, which mainly participates in cellular signal transduction and calcium ion regulation processes. This enzyme regulates the activity of key signaling pathways such as Notch and Jagged by hydroxylating specific aspartic acid residues, playing a significant role in embryonic development, cell migration and tumorigenesis. Studies have found that ASPH is abnormally highly expressed in various malignant tumors such as liver cancer and pancreatic cancer, and its tumor-promoting mechanism is closely related to the activation of invasion-related pathways. After scientists first cloned the full-length sequence of this gene in 2003, its unique hydroxylated functional domain became a new target for the development of anti-cancer drugs. In recent years, small molecule inhibitors targeting ASPH have demonstrated significant anti-tumor metastasis effects in preclinical studies, providing new ideas for cancer treatment.
Structure of ASPH
The ASPH gene encodes aspartic acid β-hydroxylase, with a protein molecular weight of approximately 86 kDa. However, there are certain differences among different species, mainly due to post-translational modifications (such as glycosylation) and the influence of shear variants.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | ~86 | ~85 | ~85.5 | ~86.2 |
| Primary Structural Differences | Contains multiple functional domains (hydroxylase, transmembrane region) | Highly conserved, with similar hydroxylase activity | With the human ASPH high homology | There are a few amino acid variations |
The ASPH protein is composed of 758 amino acids and has a typical type II transmembrane protein structure, including an N-terminal cytoplasmic tail, a transmembrane region, and a C-terminal catalytic domain. Its active center relies on Fe2+ and α-ketoglutaric acid for hydroxylation reactions, and key residues maintain enzyme activity. ASPH regulates cell migration and tumor invasion by hydroxylating specific aspartic acids of signaling proteins such as Notch and Jagged. This protein is abnormally highly expressed in liver cancer and pancreatic cancer, making it a potential therapeutic target.
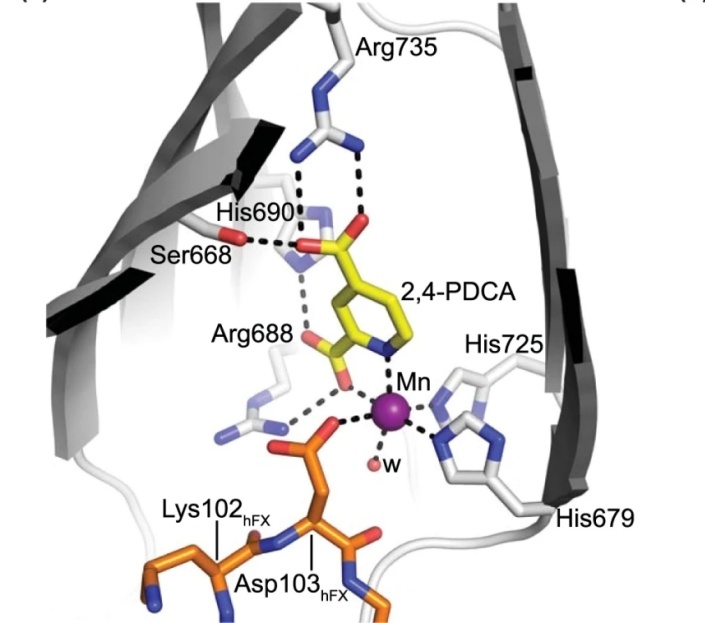 Fig. 1 Close-up of the AspH active site.1
Fig. 1 Close-up of the AspH active site.1
Key structural properties of ASPH:
- Multi-domain transmembrane proteins
- Hydroxylase activity center dependent on Fe2+/α-ketoglutarate
- Substrate identification pocket
- Oxidation-sensitive regulatory mechanism
Functions of ASPH
The main function of ASPH is to regulate cellular signal transduction through hydroxylation modification, but it also plays a key role in various pathophysiological processes.
| Function | Description |
| Notch signal activation | Hydroxylating the intracellular domain (NICD) of Notch receptors enhances its stability and transcriptional activity, promoting cell differentiation and tumorigenesis. |
| Calcium ion homeostasis regulation | Through hydroxylated calmodulin proteins (such as Jagged1), it affects the endoplasmic reticulum-mitochondrial calcium flow and participates in cell migration and invasion. |
| Promotion of tumor metastasis | t is highly expressed in liver cancer and pancreatic cancer and enhances the spread ability of cancer cells through the EMT (epithelial-mesenchymal transition) pathway. |
| Regulation of embryonic development | Mediates the hydroxylation of key proteins in the Wnt/β-catenin pathway, influencing the migration of neural crest cells and organ formation. |
| Hypoxia adaptive response | In the tumor microenvironment, its hydroxylation activity is regulated by HIF-1α, promoting angiogenesis and metabolic reprogramming. |
The enzymatic activity of ASPH shows substrate concentration-dependent (Mie kinetics) and forms a synergistic catalysis with α-ketoglutaric acid, with the highest efficiency at pH 7.4. Compared with other hydroxylases (such as PHD), the specificity of ASPH for aspartate residues makes it a potential target for cancer treatment. In preclinical models, inhibiting ASPH can significantly reduce the tumor metastasis rate.
Applications of ASPH and ASPH Antibody in Literature
1. Peng, Hui, et al. "ASPH regulates osteogenic differentiation and cellular senescence of BMSCs." Frontiers in Cell and Developmental Biology 8 (2020): 872. https://doi.org/10.3389/fcell.2020.00872
Research has found that the ASPH gene affects the osteogenic differentiation and senescence of bone marrow mesenchymal stem cells by regulating the Wnt/Gsk3β signaling pathway. Its expression decreases with age. Knockout of ASPH accelerates cellular senescence and inhibits osteogenesis, while overexpression reverses this phenomenon, suggesting that ASPH may be a new target for the treatment of age-related bone loss.
2. Lin, Qiushi, et al. "ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis." Molecular cancer 18.1 (2019): 156.https://doi.org/10.1186/s12943-019-1077-0
Research has found that ASPH promotes the synthesis and release of metastasis-promoting exosomes by activating the Notch signaling pathway, driving the invasion, metastasis and stem cell characteristics of breast cancer. ASPH is highly expressed in breast cancer and is associated with a poor prognosis. Its small molecule inhibitors can significantly inhibit metastasis, suggesting its potential as a therapeutic target.
3. Sun, Xiaojuan, et al. "ASPH Is a Metastatic Factor and Therapeutic Target in Chondrosarcoma." Cancers 17.6 (2025): 951.https://doi.org/10.3390/cancers17060951
Studies have found that ASPH is highly expressed in chondrosarcoma and is associated with a poor prognosis. Targeted inhibition of ASPH can significantly reduce tumor proliferation, invasion and MMPs secretion, and inhibit tumor growth and lung metastasis in vivo, suggesting that ASPH is a potential new target for the treatment of chondrosarcoma.
4. Holtzman, Noa G., et al. "Aspartate β-hydroxylase (ASPH) expression in acute myeloid leukemia: a potential novel therapeutic target." Frontiers in Oncology 11 (2021): 783744. https://doi.org/10.3389/fonc.2021.783744
Research has found that approximately 40% of patients with acute myeloid leukemia (AML) have abnormal expression of ASPH protein on the surface of their blasts, especially in African Americans. ASPH may serve as a new therapeutic target for AML, but its expression level has no significant correlation with clinical prognosis.
5. Lei, Cheng, et al. "Whole-exome sequencing identified a novel homozygous ASPH frameshift variant causing Traboulsi syndrome in a Chinese family." Molecular Genetics & Genomic Medicine 9.1 (2021): e1553.https://doi.org/10.1002/mgg3.1553
For the first time, a novel homozygous variation (c.1910del) of the ASPH gene was discovered in a close relative family in China, leading to Traboulsi syndrome. The clinical manifestations include new features such as lens ectopic combined with ventricular septal defect. This variation disrupts the protein function by truncating the AspH oxygenase domain, expanding the mutation spectrum of this rare disease.
Creative Biolabs: ASPH Antibodies for Research
Creative Biolabs specializes in the production of high-quality ASPH antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ASPH Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ASPH antibodies, custom preparations, or technical support, contact us at email.
Reference
- Brewitz, Lennart, et al. "Aspartate/asparagine-β-hydroxylase: a high-throughput mass spectrometric assay for discovery of small molecule inhibitors." Scientific reports 10.1 (2020): 8650. https://doi.org/10.1038/s41598-020-65123-9
Anti-ASPH antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-AMACR Recombinant Antibody (CB34A) (CBMAB-CA034LY)

-
Mouse Anti-ALDOA Recombinant Antibody (A2) (CBMAB-A2316-YC)

-
Mouse Anti-CA9 Recombinant Antibody (CBXC-2079) (CBMAB-C0131-CQ)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Mouse Anti-CRYAB Recombinant Antibody (A4345) (CBMAB-A4345-YC)

-
Mouse Anti-EIF4G1 Recombinant Antibody (2A9) (CBMAB-A2544-LY)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Mouse Anti-ACVR1C Recombinant Antibody (V2-179685) (CBMAB-A1041-YC)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-FOXL1 Recombinant Antibody (CBXF-0845) (CBMAB-F0462-CQ)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-CGAS Recombinant Antibody (CBFYM-0995) (CBMAB-M1146-FY)

-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-ATP1B1 Recombinant Antibody (E4) (CBMAB-0463-LY)

-
Mouse Anti-FeLV g27 Recombinant Antibody (1) (CBMAB-V208-1714-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








