HRAS Antibodies
Background
The HRAS gene encodes a small GTPase protein belonging to the RAS family, which mainly exists on the inner side of the cell membrane and participates in the signal transduction process that regulates cell growth and division. This protein precisely controls downstream pathways like a "molecular switch" by switching between its active state (GTP binding) and inactive state (GDP binding), thereby influencing cell proliferation and differentiation. As one of the earliest discovered oncogenes, mutations in HRAS continuously activate pro-cell growth signals, leading to the occurrence of various tumors. In 1982, scientists first identified carcinogenic mutations of HRAS in human bladder cancer cells. This breakthrough not only revealed the molecular mechanism of cancer occurrence but also laid the foundation for the subsequent development of targeted therapies. Its clear signal transduction mechanism and mutational effects have become key research objects in the fields of tumor biology and precision medicine, profoundly promoting the understanding of cell cycle regulation and carcinogenic mechanisms.
Structure of HRAS
HRAS is a small signal transduction protein with a molecular weight of approximately 21.3 kDa, and its precise molecular weight varies slightly among different splicing variants.
| Species | Human | Mouse | Rat | Zebrafish |
| Molecular Weight (kDa) | 21.3 | 21.2 | 21.3 | 21.1 |
| Primary Structural Differences | GTP combination structure domain made highly conservative | The 12th common mutation of glycine | Glutamine at position 61 is prone to mutation | The active regions of GTPase are similar |
This protein is composed of 189 amino acids, and its tertiary structure forms typical RAS family folding features: with six β -folds at the core and five α -helices surrounding it. Amino acids at positions 12, 13 and 61 constitute the key "switch regions". Mutations at these sites can disrupt the hydrolytic activity of GTP, leading to the continuous activation of the protein. The CAAX motif at its C-terminal achieves membrane localization through fanicylation modification, a feature that is crucial for the cell's signal transduction function.
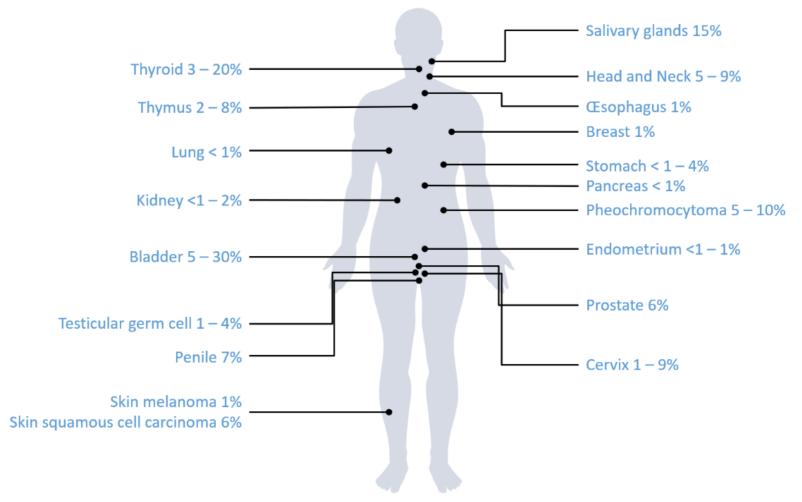 Fig. 1 Frequencies of HRAS mutations among solid cancer types.1
Fig. 1 Frequencies of HRAS mutations among solid cancer types.1
Key structural properties of HRAS:
- Typical folded conformation of the RAS family
- GTP/GDP binding pockets and their surrounding switching regions
- Highly conserved phosphate-binding ring (P-loop) domains
- Amino acids at positions 12, 13 and 61 constitute mutation hotspots
- C-terminal CAAX motifs mediate membrane localization and signal transduction
Functions of HRAS
The main function of HRAS protein is to act as a molecular switch in cellular signal transduction. However, it is also involved in regulating various cellular physiological processes, including proliferation, differentiation and survival.
| Function | Description |
| Gtpase activity | HRAS achieves molecular switch function by hydrolyzing GTP to GDP and circulates between active and inactive states. |
| Signal transduction regulation | As a key node of the MAPK/ERK pathway, it transmits growth factor signals from membrane receptors to the nucleus. |
| Cell cycle promotion | Activated HRAS promotes the progression from G1 to S phases by regulating the expression of cyclins such as cyclin D1. |
| Anti-apoptotic effect | Inhibit cell apoptosis and enhance cell survival ability through the PI3K-AKT pathway. |
| Metabolic reprogramming | Induce the expression of glycolycle-related genes to support the Warburg effect of tumor cells. |
The affinity of HRAS for GTP exhibits typical single-molecule binding characteristics, in contrast to the synergistic effect of large signal complexes, which reflects its high response characteristics as a fundamental signal hub.
Applications of HRAS and HRAS Antibody in Literature
1. Mathiot, Laurent, et al. "HRAS Q61L mutation as a possible target for non-small cell lung cancer: Case series and review of literature." Current Oncology 29.5 (2022): 3748-3758. https://doi.org/10.3390/curroncol29050300
The article indicates that in non-small cell lung cancer, the incidence of HRAS p.glln61LEU mutation is approximately 0.25%, which is more common in smokers and often accompanied by pleural or pericardial effusion, with a poor prognosis. The current therapies have limited effects. The farnesyltransferase inhibitor tipifarnib may offer a new treatment direction for patients with this mutation.
2. Bièche, Ivan, et al. "HRAS is a therapeutic target in malignant chemo-resistant adenomyoepithelioma of the breast." Journal of Hematology & Oncology 14.1 (2021): 143. https://doi.org/10.1186/s13045-021-01158-3
Studies have found that hotspot mutations of HRAS G13R or G12S are common in malignant adenomyoepitheliomas of the breast. Patient-derived transplanted tumor models have confirmed that the MEK inhibitor trametinib has a significant therapeutic effect on this type of HRAS-mutated tumors, providing a potential treatment direction for advanced patients.
3. Lü, Guohua, et al. "LINC00623/miR-101/HRAS axis modulates IL-1β-mediated ECM degradation, apoptosis and senescence of osteoarthritis chondrocytes." Aging (Albany NY) 12.4 (2020): 3218. https://doi.org/10.18632/aging.102801
Research has found that HRAS is down-regulated in osteoarthritis. HRAS alleviates chondrocyte apoptosis and extracellular matrix degradation through the LINC00623/miR-101 molecular axis, thereby delaying the progression of osteoarthritis. This mechanism provides new potential targets for treatment.
4. Lindsey-Temple, Suzanna, et al. "A novel HRAS c. 466C> T p.(Phe156Leu) variant in two patients with attenuated features of Costello syndrome." European Journal of Human Genetics 30.9 (2022): 1088-1093. https://doi.org/10.1038/s41431-022-01139-1
The report found that the novel P. he156Leu mutation of HRAS can lead to Costrol syndrome. The patient presented with typical symptoms such as feeding difficulties and developmental delay, but the facial and skin features were relatively mild. Functional studies have shown that this mutation enhances downstream signal transduction, confirming the diversity of the pathogenic mechanisms of HRAS mutations.
5. Pareja, Fresia, et al. "Immunohistochemical assessment of HRAS Q61R mutations in breast adenomyoepitheliomas." Histopathology 76.6 (2020): 865-874. https://doi.org/10.1111/his.14057
This study evaluated the efficacy of RAS Q61R immunohistochemistry in detecting HRAS Q61R mutations in adenomyoepithelioma of the breast. The results show that this technique has a specificity of 100% and a sensitivity of 71%. It can effectively identify specific mutations in ER-negative cases and is a practical auxiliary diagnostic method.
Creative Biolabs: HRAS Antibodies for Research
Creative Biolabs specializes in the production of high-quality HRAS antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom HRAS Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our HRAS antibodies, custom preparations, or technical support, contact us at email.
Reference
- Mathiot, Laurent, et al. "HRAS Q61L mutation as a possible target for non-small cell lung cancer: Case series and review of literature." Current Oncology 29.5 (2022): 3748-3758. https://doi.org/10.3390/curroncol29050300
Anti-HRAS antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ENO1 Recombinant Antibody (CBYC-A950) (CBMAB-A4388-YC)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-FOSB Recombinant Antibody (CBXF-3593) (CBMAB-F2522-CQ)

-
Mouse Anti-CCN2 Recombinant Antibody (CBFYC-2383) (CBMAB-C2456-FY)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-dsDNA Recombinant Antibody (22) (CBMAB-AP1954LY)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Mouse Anti-CCL18 Recombinant Antibody (64507) (CBMAB-C7910-LY)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Rat Anti-EMCN Recombinant Antibody (28) (CBMAB-E0280-FY)

-
Mouse Anti-DLL4 Recombinant Antibody (D1090) (CBMAB-D1090-YC)

-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-CALR Recombinant Antibody (CBFYC-0763) (CBMAB-C0818-FY)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






