PAX6 Antibodies
Background
PAX6 gene as a key transcription factor, mainly exist in the vertebrate eyes, brain and pancreas, the organ from which the. This gene participates in the formation of ocular structures and the differentiation of the nervous system during embryonic development by regulating the expression of downstream target genes, and plays a core role in maintaining normal visual function. The PAX6 homologous gene of invertebrates (if flies) also exhibits a similar function, indicating that it is highly conserved evolutionarily. First discovered by Walter Gehring's team in 1991, PAX6 is recognized as the master gene for eye development, and its mutations can lead to hereditary eye diseases such as congenital aniritis in humans. This gene has been widely studied due to its paradigmatic role in developmental biology, providing an important model for understanding gene regulatory networks, cell fate determination, and organogenesis mechanisms.
Structure of PAX6
PAX6 is a transcription factor protein with a molecular weight of approximately 46 kDa, and its precise molecular weight varies slightly among different species. The following table lists the molecular weight and main structural characteristics of PAX6 protein in different species:
| Species | Human | Mouse | Fruit fly | Zebrafish |
| Molecular Weight (kDa) | 46.2 | 45.9 | 48.3 | 45.6 |
| Primary Structural Differences | Contains PD domain and Homeodomain with transcriptional activation domain | PAX6 highly homologous with humans, the domain of similar structure | Retain the PD domain and Homeodomain, but the C-end sequence is different | Has two DNA binding domain structure, but the transcriptional activation regional variation |
The PAX6 protein is composed of two highly conserved DNA-binding domains: the N-terminal PD domain (128 amino acids) and the C-terminal Homeodomain (60 amino acids). These two domains form stable three-dimensional structures through specific protein folding, enabling them to recognize and bind to specific DNA sequences. The C-terminal of the protein contains a transcriptional activation region rich in proline, serine and threonine, which plays a key role in the regulation of gene expression. PAX6 binds to the promoter regions of target genes through its DNA-binding domain, thereby regulating the expression of genes related to ocular development and neural differentiation.
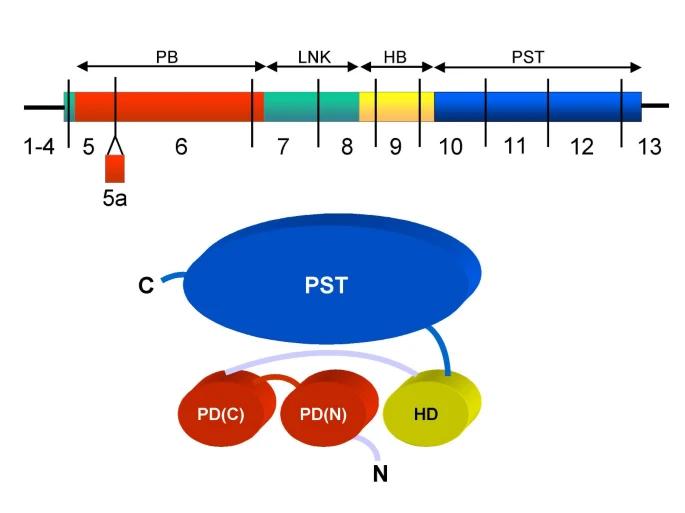 Fig. 1 The PAX6 cDNA and protein.1
Fig. 1 The PAX6 cDNA and protein.1
Key structural properties of PAX6:
- Contains two DNA binding domains: PD domain and homeotypic domain
- Highly conserved helical-rotation helical motifs are used for specific DNA recognition
- The C-terminal transcriptional activation region is rich in proline, serine and threonine
Functions of PAX6
The main function of the PAX6 gene is to regulate the formation of the eyes and nervous system during embryonic development. In addition, it is also involved in the development of the pancreas and the cell differentiation of sensory organs.
| Function | Description |
| Regulation of ocular development | As a master gene regulating the formation of the eyeball, lens and retina, mutations lead to congenital eye diseases. |
| Differentiation of the nervous system | Participate in the development and regional specialization of cerebral cortex, spinal cord and olfactory neurons. |
| Specialization of pancreatic cells | Effect of islet β cells differentiation and insulin secretion. |
| Regulation of gene expression | Activate or inhibit downstream target genes by binding to specific DNA sequences. |
| Evolutionary conservation | From fruit flies to mammals are dominant in the visual system development, a highly conservative evolution. |
PAX6 collaboratively recognizes and binds to the enhancer regions of target genes through its PD domain and Homeodomain. Its binding specificity is higher than that of many universal transcription factors, demonstrating its precise regulatory function during development.
Applications of PAX6 and PAX6 Antibody in Literature
1. Polisetti, Naresh, Günther Schlunck, and Thomas Reinhard. "PAX6 expression patterns in the adult human limbal stem cell niche." Cells 12.3 (2023): 400. https://doi.org/10.3390/cells12030400
In this study, through immunohistochemistry and Western blot, it was found that PAX6 was only expressed in limbal epithelial progenitor cells (LEPC), but not in stromal cells (LMSC) or melanocytes, indicating that the expression of PAX6 was specifically confined to the epithelial cells in the limbal stem cell microenvironment.
2. Ochi, Shohei, et al. "Thirty years' history since the discovery of Pax6: from central nervous system development to neurodevelopmental disorders." International Journal of Molecular Sciences 23.11 (2022): 6115. https://doi.org/10.3390/ijms23116115
The article indicates that Pax6 is a key transcription factor that regulates the formation of brain region patterns, proliferation and differentiation of neural stem cells during the development of the nervous system. This gene mutation is associated with WAGR syndrome, autism spectrum disorder and intellectual disability, and is of great significance for studying the mechanism of neurodevelopmental diseases.
3. Yu, Fei, et al. "PAX6, modified by SUMOylation, plays a protective role in corneal endothelial injury." Cell Death & Disease 11.8 (2020): 683. https://doi.org/10.1038/s41419-020-02848-5
The article indicates that PAX6 is upregulated after corneal endothelial injury and alleviates the injury by enhancing the tight junctions of cells. Sumoylation modification (at K53/K89 sites) can reduce the stability of PAX6, while SENP1-mediated desumoylation can enhance the stability of PAX6 protein and promote endothelial repair, suggesting its potential as a therapeutic target.
4. Shohayeb, Belal, and Helen M. Cooper. "The ups and downs of Pax6 in neural stem cells." Journal of Biological Chemistry 299.5 (2023). https://doi.org/10.1016/j.jbc.2023.104680
Research has found that the acetylation modification of Pax6 protein mediated by Kat2a can promote its ubiquitination and degradation through the proteasome, thereby regulating the proliferation and differentiation process of neural stem cells. This new mechanism reveals the post-translational regulatory mode at the Pax6 protein level.
5. Hou, Ya-Nan, Sheng Li, and Yun-**a Luan. "Pax6 in collembola: adaptive evolution of eye regression." Scientific Reports 6.1 (2016): 20800. https://doi.org/10.1038/srep20800
In this study, by cloning the Pax6 genes of both eyeless and eyeless jumping bugs, it was found that both could induce the formation of ectopic eyes in fruit flies. However, the activation ability of Fc-Pax6 in soil eyeless jumping bugs was significantly weakened due to mutations at the key C-terminal site. This proved at the molecular level that the monophyletic origin and degeneration of jumping bug eyes are due to adaptive evolution.
Creative Biolabs: PAX6 Antibodies for Research
Creative Biolabs specializes in the production of high-quality PAX6 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom PAX6 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our PAX6 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Tzoulaki, Ioanna, Ian MS White, and Isabel M. Hanson. "PAX6 mutations: genotype-phenotype correlations." BMC genetics 6.1 (2005): 27. https://doi.org/10.1186/1471-2156-6-27
Anti-PAX6 antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-4-Hydroxynonenal Recombinant Antibody (V2-502280) (CBMAB-C1055-CN)

-
Mouse Anti-ADGRE2 Recombinant Antibody (V2-261270) (CBMAB-C0813-LY)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-ATG5 Recombinant Antibody (9H197) (CBMAB-A3945-YC)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-AMOT Recombinant Antibody (CBYC-A564) (CBMAB-A2552-YC)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Rat Anti-CD300A Recombinant Antibody (172224) (CBMAB-C0423-LY)

-
Mouse Anti-ENO2 Recombinant Antibody (85F11) (CBMAB-0276CQ)

-
Rat Anti-AChR Recombinant Antibody (V2-12500) (CBMAB-0990-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






