RUNX2 Antibodies
Background
RUNX2, as a core transcriptional regulatory protein, is mainly expressed in osteoblasts and chondrocytes. This gene dominates the process of bone matrix formation and mineralization by regulating the transcriptional activity of downstream target genes, playing a decisive role in bone development and homeostasis maintenance. In mammals, mutations in RUNX2 can lead to hereditary bone diseases such as hypoplasia of the skull and clavicle. This gene was first identified by the team of Japanese scientist Toshihisa Komori in 1997. Its three-dimensional structure was analyzed by X-ray crystallography in 2004, revealing the specific mechanism by which the RUNT domain binds to DNA. As a key molecular switch in bone formation, RUNX2's multi-level regulatory network has become an important target for the treatment of bone diseases and regenerative medicine research.
Structure of RUNX2
Myoglobin is a relatively small protein with a molecular weight of approximately 16.7 kDa. This weight may slightly vary between species due to minor differences in amino acid sequence.
| Species | Human | Mouse | Rat | Bovine | Zebrafish |
| Molecular Weight (kDa) | 55.6 | 55.3 | 55.5 | 55.8 | 54.9 |
| Primary Structural Differences | Contains 521 amino acids | 518 amino acids | 519 amino acids | 523 amino acids | 507 amino acids |
The RUNX2 protein contains the N-terminal RUNX domain, the C-terminal transcriptional activation domain, and the nuclear localization signal. Its tertiary structure shows that the RUNX domain adopts a β -barrel folding mode and, after binding with the CBFβ cofactor to form a heterodimer, enhances the DNA binding ability. This protein regulates the transcription of target genes by specifically recognizing the core sequence 5'-ACCACA-3', and its C-terminal proline/serine/threonine enrichment region mediates the transcriptional activation function. This multi-domain synergy enables RUNX2 to precisely regulate the expression of genes related to osteoblast differentiation and bone development.
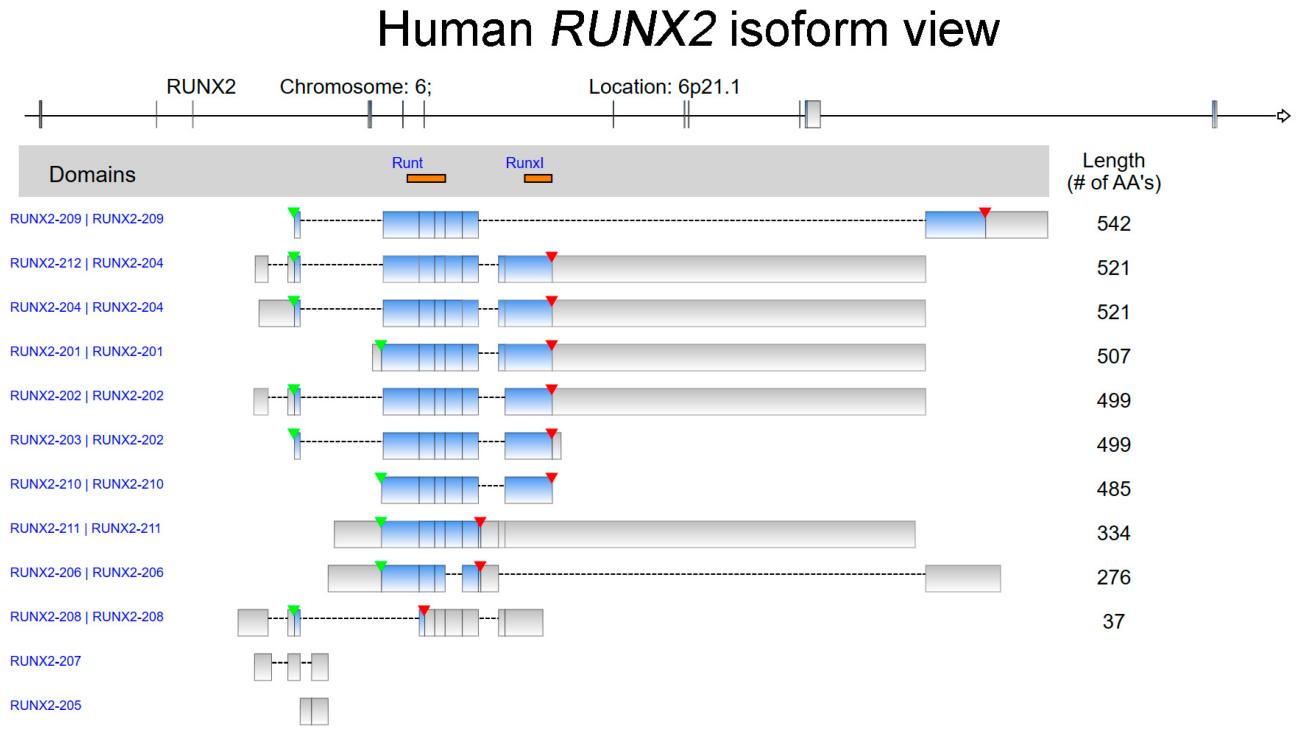 Fig. 1 The isoform view of human RUNX2.1
Fig. 1 The isoform view of human RUNX2.1
Key structural properties of RUNX2:
- Contains a conserved Runt domain that mediates DNA binding
- The C-terminal transcriptional activation domain is rich in proline/serine/threonine
- Nuclear localization signals ensure the aggregation of proteins within the nucleus
Functions of RUNX2
The main function of the RUNX2 gene is to regulate bone development and osteoblast differentiation. In addition, it is also involved in a variety of biological processes, including cell fate determination and disease occurrence.
| Function | Description |
| Regulation of bone formation | RUNX2 activates the expression of osteogenic specific genes and dominates the process of bone matrix synthesis and mineralization. |
| Chondrocyte differentiation | Regulate the process of endochondral osteogenesis and promote the maturation and calcification of chondrocytes. |
| Directed differentiation of stem cells | Ectomesenchymal stem cell decided to osteogenesis cell differentiation, inhibit fat forming path. |
| Odontogenesis | Participate in tooth germ development and dentine formation, regulation of tooth source sex differentiation. |
| Promotion of cancer metastasis | Abnormal expression in breast and prostate cancer, and promote the process of bone metastases. |
RUNX2 exerts transcriptional activation by binding to the ACCACA sequence in the promoter region of the target gene. Its activity is precisely regulated by multiple signaling pathways such as BMP/Smad and Wnt/β-catenin, playing a core role in maintaining bone homeostasis and pathological processes.
Applications of RUNX2 and RUNX2 Antibody in Literature
1. Lin, Tsung-Chieh. "RUNX2 and Cancer." International Journal of Molecular Sciences 24.8 (2023): 7001. https://doi.org/10.3390/ijms24087001
The article indicates that RUNX2, as a key transcription factor, not only regulates cell differentiation, but also its somatic mutations and expression characteristics are associated with the progression, metastasis and drug resistance of various cancers. It is regarded as a potential cancer biomarker. Recent studies have revealed its mechanism and clinical significance in tumorigenesis.
2. Komori, Toshihisa. "Regulation of skeletal development and maintenance by Runx2 and Sp7." International Journal of Molecular Sciences 25.18 (2024): 10102. https://doi.org/10.3390/ijms251810102
The article indicates that Runx2 is a core transcription factor in bone development. It regulates the orientation, proliferation and differentiation of mesenchymal cells towards the osteoblast lineage, and induces the expression of bone matrix protein genes. Runx2 interacts with multiple signaling pathways, such as Hedgehog and Wnt, and coordinates the osteogenesis process by inducing factors like Sp7. In addition, Runx2 is also an important pathogenic factor in osteoarthritis.
3. Gargalionis, Antonios N., et al. "Runx2 and polycystins in bone mechanotransduction: challenges for therapeutic opportunities." International Journal of Molecular Sciences 25.10 (2024): 5291. https://doi.org/10.3390/ijms25105291
The article indicates that Runx2 is a core molecule in bone mechanical conduction, regulated by mechanical stimuli and collaborating with factors such as polycystic proteins to dominate the process of bone remodeling and regeneration. Its targeting strategy provides a new direction for the treatment of bone loss diseases.
4. Błasiak, Janusz, Daniel Wysokiński, and Elżbieta Pawłowska. "Role of RUNX2 in Breast Carcinogenesis." International Journal of Molecular Sciences 16.9 (2015). https://doi.org/10.3390/ijms160920969
The article indicates that RUNX2 is not only a key factor for osteogenesis but also promotes the development of breast cancer by regulating DNA damage response, cell cycle and estrogen signaling pathways, especially closely related to the interaction between the Wnt/Tgfβ pathway and ERα, and can serve as a potential therapeutic target.
5. Wang, Cheng, et al. "Poly (ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2." Nature communications 10.1 (2019): 1203. https://doi.org/10.1038/s41467-019-09174-1
The article indicates that PARP1 activity increases in vascular calcification. It inhibits the expression of miR-204 through the IL-6/STAT3 pathway, reliefs the inhibition of Runx2, thereby promoting the expression of Runx2 protein and the transdifferentiation of vascular smooth muscle cells into osteogenic phenotypes, and intensifying calcium deposition.
Creative Biolabs: RUNX2 Antibodies for Research
Creative Biolabs specializes in the production of high-quality RUNX2 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom RUNX2 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our RUNX2 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Lin, Tsung-Chieh. "RUNX2 and Cancer." International Journal of Molecular Sciences 24.8 (2023): 7001. https://doi.org/10.3390/ijms24087001
Anti-RUNX2 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ATG5 Recombinant Antibody (9H197) (CBMAB-A3945-YC)

-
Mouse Anti-C5AR1 Recombinant Antibody (R63) (CBMAB-C9553-LY)

-
Mouse Anti-BANF1 Recombinant Antibody (3F10-4G12) (CBMAB-A0707-LY)

-
Mouse Anti-ACE2 Recombinant Antibody (V2-179293) (CBMAB-A0566-YC)

-
Mouse Anti-4-Hydroxynonenal Recombinant Antibody (V2-502280) (CBMAB-C1055-CN)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Mouse Anti-ASB9 Recombinant Antibody (1D8) (CBMAB-A0529-LY)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-CD164 Recombinant Antibody (CBFYC-0077) (CBMAB-C0086-FY)

-
Mouse Anti-EIF4G1 Recombinant Antibody (2A9) (CBMAB-A2544-LY)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Mouse Anti-ADGRE5 Recombinant Antibody (V2-360335) (CBMAB-C2088-CQ)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-EMP3 Recombinant Antibody (CBFYE-0100) (CBMAB-E0207-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








