VWA2 Antibodies
Background
VWA2 is A protein containing the von Willebrand factor A domain, mainly existing in various tissues of vertebrates, especially playing an important role in the extracellular matrix and cell adhesion processes. This protein participates in key physiological processes such as tissue development, wound repair and cell signal transduction by mediating the interaction between cells and the matrix. VWA2 was first identified in 2004. Its structural features are highly similar to those of the cell adhesion molecule family, but the specific functional mechanism is still under in-depth study. As an important component of the extracellular matrix, the unique structure of VWA2 provides scientists with a new perspective for studying pathological processes such as cell migration and tumor metastasis. In recent years, it has gradually become a hot molecule in cell biology and medical research.
Structure of VWA2
VWA2 is an extracellular matrix protein with a molecular weight of approximately 50-60 kDa. Its precise molecular weight may vary slightly due to differences in amino acid sequences among different species.
| Species | Human | Mice | Rats |
| Molecular Weight (kDa) | 55-60 | 54-58 | 56-59 |
| Primary Structural Differences | Contains vWA domain, involved in cell adhesion | Highly homologous to humans and functionally conserved | Similar to human VWA2, but with some sequence differences |
VWA2 is composed of multiple functional domains, among which the von Willebrand factor A (vWA) domain mediates protein interactions, especially playing a key role in extracellular matrix (ECM) assembly and cell migration. The three-dimensional structure of this protein contains multiple β -folds and α -helices, forming a stable binding interface that enables it to interact with molecules such as collagen and integrins. VWA2 plays a regulatory role in various physiological and pathological processes, such as cancer metastasis and tissue repair, and is an important target for the study of the cellular microenvironment.
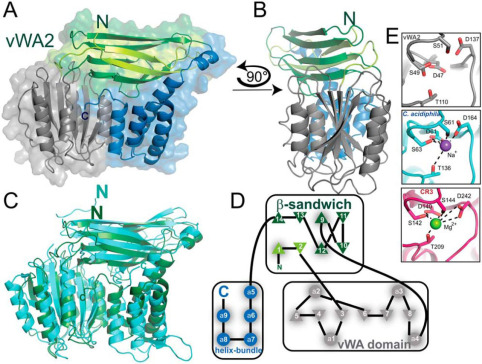 Fig. 1 Crystal structure of vWA2.1
Fig. 1 Crystal structure of vWA2.1
Key structural properties of VWA2:
- Modular configuration with vWA domain
- Hydrophobic and hydrophilic regions are alternately distributed
- Conservative disulfide bond network
- Integrin binding motif
Functions of VWA2
VWA2 is an extracellular matrix protein that mainly participates in cell-matrix interactions and tissue structure maintenance, and plays a key role in a variety of pathophysiological processes.
| Function | Description |
| Regulation of cell adhesion | Mediated by vWA structure domain cells and collagen, and laminin matrix components interaction, affect the cell migration and positioning. |
| Maintenance of organizational barriers | Participate in the assembly of the basement membrane and maintain the structural integrity and selective permeability of the epithelial/endothelial cell layer. |
| Regulation of the tumor microenvironment | Abnormal expression in a variety of cancers (such as colon cancer, breast cancer), affecting tumor metastasis and angiogenesis. |
| Participation in wound repair | By regulating fibroblast activity, it promotes extracellular matrix remodeling and tissue regeneration. |
| Immune regulatory function | Interacts with integrins to affect leukocyte migration and inflammatory response. |
The mode of action of VWA2 shows microenvironmental dependence: in normal tissues, it mainly plays a structural support role, while in pathological conditions (such as the tumor microenvironment), it may release biologically active fragments through proteinase-mediated proteolysis to participate in signal transduction regulation. Its functional realization relies on the unique metal ion-dependent adhesion sites (MIDAS) of the vWA domain, a feature that makes it a key regulatory molecule in the dynamic remodeling process of the extracellular matrix.
Applications of VWA2 and VWA2 Antibody in Literature
1. van der Ven, Amelie T., et al. "A homozygous missense variant in VWA2, encoding an interactor of the Fraser-complex, in a patient with vesicoureteral reflux." PloS one 13.1 (2018): e0191224. https://doi.org/10.1371/journal.pone.0191224
The research found that through whole exome sequencing and homozygous localization, a homozygous missense mutation (p.Arg446Cys) of the VWA2 gene was identified in a close relative family in India. This mutation alters protein transport through abnormal disulfide bond formation and is associated with CAKUT. Immunohistochemistry revealed that VWA2 and Fras1 were co-located in the nephron-occurring region, suggesting their potential as novel pathogenic genes for CAKUT.
2. Gonzalez, Beatriz, et al. "Epigenetic and transcriptional dysregulation of VWA2 associated with a MYC-driven oncogenic program in colorectal cancer." Scientific Reports 8.1 (2018): 11097. https://doi.org/10.1038/s41598-018-29378-7
Research has found that the VWA2 gene is significantly upregulated in colorectal cancer (CRC), with high-frequency hypomethylation in its downstream region accompanied by histone modification changes. The expression of this gene is significantly correlated with the c-Myc target gene. In 78% of CRC cases, the expression increases by more than 15 times, and it has the potential as a specific biomarker.
3. Hoffmann, Lena, et al. "Structure and interactions of the archaeal motility repression module ArnA–ArnB that modulates archaellum gene expression in Sulfolobus acidocaldarius." Journal of Biological Chemistry 294.18 (2019): 7460-7471. https://doi.org/10.1074/jbc.RA119.007709
Studies have found that in the acidophilic archaea Sulfolobus acidocaldarius, the vWA2 domain protein ArnB and the FHA domain protein ArnA interact through phosphorylation-dependent interaction, synergically regulating the flagellar movement of the archaea. Crystal structure analysis reveals that vWA2 contains a complex topological structure, and its C-terminal phosphorylation site mediates nutritional condition-dependent functional regulation.
4. Gebauer, Jan M., et al. "O-glucosylation and O-fucosylation occur together in close proximity on the first epidermal growth factor repeat of AMACO (VWA2 protein)." Journal of Biological Chemistry 283.26 (2008): 17846-17854. https://doi.org/10.1074/jbc.M704820200
Research has found that the extracellular matrix protein AMACO (VWA2) contains VWA and EGF-like domains, and its EGF domain can undergo both O-glucosylation and O-fucosylation modifications simultaneously. This protein is specifically expressed in the basement membranes of the skin, lungs and kidneys, may be involved in structural support or cell anchoring, and is significantly upregulated in colon cancer, having potential diagnostic value.
5. Esho, Temitope, et al. "The Fraser Complex Proteins (Frem1, Frem2, and Fras1) Can Form Anchoring Cords in the Absence of AMACO at the Dermal–Epidermal Junction of Mouse Skin." International Journal of Molecular Sciences 24.7 (2023): 6782. https://doi.org/10.3390/ijms24076782
Research has found that the basement membrane protein AMACO (VWA2) is highly expressed during embryonic development and co-locates with the Fraser complex to form an anchoring cable structure. However, gene knockout experiments showed that the deletion of AMACO did not affect the deposition of the Fraser complex or the function of the anchoring cable, indicating that it is not an essential protein for development.
Creative Biolabs: VWA2 Antibodies for Research
Creative Biolabs specializes in the production of high-quality VWA2 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom VWA2 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our VWA2 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Hoffmann, Lena, et al. "Structure and interactions of the archaeal motility repression module ArnA–ArnB that modulates archaellum gene expression in Sulfolobus acidocaldarius." Journal of Biological Chemistry 294.18 (2019): 7460-7471. https://doi.org/10.1074/jbc.RA119.007709
Anti-VWA2 antibodies
 Loading...
Loading...
Hot products 
-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-ADV Recombinant Antibody (V2-503423) (CBMAB-V208-1364-FY)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Mouse Anti-ENO1 Recombinant Antibody (8G8) (CBMAB-E1329-FY)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Mouse Anti-CHRNA9 Recombinant Antibody (8E4) (CBMAB-C9161-LY)

-
Rat Anti-ADGRE4 Recombinant Antibody (V2-160163) (CBMAB-F0011-CQ)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-1728) (CBMAB-2077-YY)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Mouse Anti-CTCF Recombinant Antibody (CBFYC-2371) (CBMAB-C2443-FY)

-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-ENO2 Recombinant Antibody (85F11) (CBMAB-0276CQ)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot