AHSP Antibodies
Background
AHSP is a small molecule chaperone protein encoded by a specific gene, mainly existing in the red blood cells of vertebrates. This protein maintains the stability and function of hemoglobin by specifically binding to free α-hemoglobin, preventing its oxidation, aggregation and precipitation, and ensuring the normal oxygen transport capacity of red blood cells. During the process of red blood cell differentiation, AHSP is crucial for maintaining the balance of α- and β-hemoglobin chains, and its deficiency may lead to pathological conditions such as hemolytic anemia. This gene was first identified in 2002. Its three-dimensional structure and interaction mechanism were analyzed through X-ray crystallography and other techniques, providing a key molecular basis for the study of hemoglobinopathies and significantly advancing our understanding of protein homeostasis, heme protection and the mechanisms of blood diseases.
Structure of AHSP
Myoglobin is a relatively small protein with a molecular weight of approximately 16.7 kDa. This weight may slightly vary between species due to minor differences in amino acid sequence.
| Species | Human | Mouse | Rhesus monkey | Rat |
| Molecular Weight (kDa) | 10.8 | 10.7 | 10.8 | 10,7 |
| Primary Structural Differences | It contains 3 α -helical domains | 90% homology with human source | Highly consistent with the human sequence | With conservative binding interface |
This protein is composed of 102 amino acids, and its secondary structure is mainly made up of three α -helices (helices 1-3), which are connected through flexible ring regions to form hydrophobic binding pockets. AHSP specifically binds to free α -hemoglobin through its C-terminal helix. Key residues such as Leu75 and Gln79 play a stabilizing role in the binding interface, effectively preventing the oxidative denaturation and precipitation of α -hemoglobin and maintaining the biological activity of hemoglobin. The three-dimensional structure of AHSP was analyzed by X-ray crystallography, revealing the structural basis of its molecular chaperone mechanism.
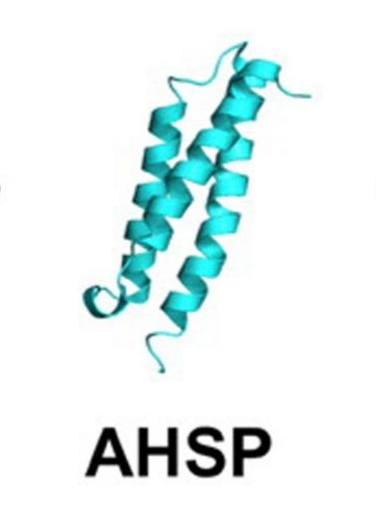 Fig. 1 The structure of AHSP.1
Fig. 1 The structure of AHSP.1
Key structural properties of AHSP:
- Compact triple helix bundle structure
- Hydrophobic core surrounded by alpha hemoglobin combined interface
- Hydrophobic pockets that specifically bind to free α -hemoglobin
Functions of AHSP
The main function of AHSP is to maintain the stability and solubility of hemoglobin in red blood cells. In addition, it is also involved in a variety of pathophysiological processes, including red blood cell differentiation, oxidative stress response and the mechanism of anemia occurrence.
| Function | Description |
| Stable hemoglobin | Specifically bind to free α -hemoglobin to prevent its denaturation, precipitation and the formation of inclusion bodies. |
| Protection against oxidative damage | Inhibit the oxidation of heme iron and the generation of free radicals, protecting cells from oxidative stress damage. |
| Red blood cell maturation support | Promote the correct assembly of α- and β -hemoglobin chains to ensure the normal development of red blood cells. |
| Adaptation to hypoxia | Maintain hemoglobin function under hypoxic conditions to support the balance of tissue oxygen supply. |
| Association of disease mechanisms | Abnormal expression of AHSP is closely related to various hemoglobinopathies such as thalassemia. |
AHSP functions through the molecular chaperone mechanism, and its binding kinetics shows a high-affinity characteristic, which is different from the synergistic effect of general chaperone proteins, highlighting its unique role in maintaining protein homeostasis within the red blood cell system.
Applications of AHSP and AHSP Antibody in Literature
1. Zurlo, Matteo, et al. "Increased Expression of α-Hemoglobin Stabilizing Protein (AHSP) mRNA in Erythroid Precursor Cells Isolated from β-Thalassemia Patients Treated with Sirolimus (Rapamycin)." Journal of Clinical Medicine 13.9 (2024): 2479. https://doi.org/10.3390/jcm13092479
This study explored the effect of sirolimus (rapamycin) on the expression of α -hemoglobin stabilizing protein (AHSP) in erythroid precursor cells of patients with β -thalassemia. The results showed that the expression of AHSP in patients was higher than that in healthy people and was further induced to increase by sirolimus, suggesting that AHSP could be used as an endpoint indicator in related clinical trials.
2. Mollan, Todd L., et al. "Kinetics of α-globin binding to α-hemoglobin stabilizing protein (AHSP) indicate preferential stabilization of hemichrome folding intermediate." Journal of Biological Chemistry 287.14 (2012): 11338-11350. https://doi.org/10.1074/jbc.m111.313247
The article indicates that AHSP is a molecular chaperone protein that can rapidly bind to and stabilize free α -globin, especially having a higher affinity for the high-iron form (met-α), forming a relatively stable complex, preventing abnormal hemoglobin assembly, and promoting normal hemoglobin production after reduction.
3. Han, Gai, et al. "Nrf2 expands the intracellular pool of the chaperone AHSP in a cellular model of β-thalassemia." Redox biology 50 (2022): 102239. https://doi.org/10.1016/j.redox.2022.102239
The article indicates that AHSP is upregulated in response to excessive α -globin through the Nrf2 pathway in β -thalassemia, which can inhibit α -globin precipitation and ROS production, and improve oxidative stress and protein aggregation during red blood cell differentiation.
4. Walczak, Julia, Maria D. Camargo Johnson, and Kuzhali Muthumalaiappan. "Stage specific expression pattern of alpha-hemoglobin-stabilizing-protein (AHSP) portrayed in erythroblast chronology." Methods and Protocols 3.3 (2020): 46. https://doi.org/10.3390/mps3030046
The article indicates that the expression of AHSP changes dynamically during the maturation of erythroid cells: it is low in the early stage, reaches a peak in the middle stage (8-12 days of culture), and decreases in the late stage, mainly located in the nucleus and excreted along with the nucleus, suggesting that its expression is stage-specific.
5. Dickson, Claire F., et al. "α-Hemoglobin-stabilizing protein (AHSP) perturbs the proximal heme pocket of oxy-α-hemoglobin and weakens the iron-oxygen bond." Journal of Biological Chemistry 288.27 (2013): 19986-20001. https://doi.org/10.1074/jbc.M112.437509
The article indicates that AHSP, as a molecular chaperone, binds to α -hemoglobin (αHb), alters the structure of its heme pockets, stretching the Fe-O₂ bond and promoting O₂ dissociation, thereby accelerating the auto-oxidation of αHb and stabilizing it in an antioxidant state when βHb is insufficient.
Creative Biolabs: AHSP Antibodies for Research
Creative Biolabs specializes in the production of high-quality AHSP antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom AHSP Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our AHSP antibodies, custom preparations, or technical support, contact us at email.
Reference
- Mollan, Todd L., et al. "Kinetics of α-globin binding to α-hemoglobin stabilizing protein (AHSP) indicate preferential stabilization of hemichrome folding intermediate." Journal of Biological Chemistry 287.14 (2012): 11338-11350. https://doi.org/10.1074/jbc.m111.313247
Anti-AHSP antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ENO1 Recombinant Antibody (8G8) (CBMAB-E1329-FY)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Rat Anti-CD300A Recombinant Antibody (172224) (CBMAB-C0423-LY)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Rabbit Anti-AP2M1 (Phosphorylated T156) Recombinant Antibody (D4F3) (PTM-CBMAB-0610LY)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-AKT1 (Phosphorylated S473) Recombinant Antibody (V2-505430) (PTM-CBMAB-0067LY)

-
Mouse Anti-2C TCR Recombinant Antibody (V2-1556) (CBMAB-0951-LY)

-
Mouse Anti-DHFR Recombinant Antibody (D0821) (CBMAB-D0821-YC)

-
Mouse Anti-ASB9 Recombinant Antibody (1D8) (CBMAB-A0529-LY)

-
Mouse Anti-CARD11 Recombinant Antibody (CBFYC-0811) (CBMAB-C0866-FY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Mouse Anti-HTLV-1 gp46 Recombinant Antibody (CBMW-H1006) (CBMAB-V208-1154-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






