ALAD Antibodies
Background
The ALAD gene encodes δ-aminolevulinic acid dehydratase (ALAD), a metalloenzyme that plays a key role in the heme biosynthesis pathway and is mainly distributed in the liver and bone marrow hematopoietic tissues. This enzyme catalyzes the condensation of two molecules of δ-aminolevulinic acid (ALA) to form bilirubin, providing a basic raw material for the subsequent synthesis of hemoglobin and is crucial for maintaining oxygen transport and energy metabolism in the body. Research has found that the polymorphism of the ALAD gene is associated with lead poisoning susceptibility, and its active site is highly sensitive to lead ions, making this gene an important biomarker in environmental toxicology research. As the second rach-limiting enzyme in the heme synthesis pathway, ALAD's biochemical mechanism was elucidated by the Shemin and Russell team in the 1950s, and its crystal structure was determined in the 1990s, providing a molecular basis for understanding the catalytic mechanism of metalloenzymes and the toxicity of heavy metals.
Structure of ALAD
ALAD is a metalloenzyme with a molecular weight of approximately 36 kDa, and the precise molecular weight varies slightly among species, mainly depending on small changes in the amino acid sequence.
| Species | Human | Mouse | Rat | Bovine | Yeast |
| Molecular Weight (kDa) | 60 | 35.8 | 35.9 | 36.2 | 37.1 |
| Primary Structural Differences | Zinc-containing binding sites, highly conserved | Highly homologous to human ALAD | Similar to a mouse, slightly modified | Tiny difference between the mammals | Eukaryotic lower organisms have slightly different structures |
The ALAD protein is composed of approximately 330 amino acids, forming an octamer structure. Each subunit contains a zinc ion binding site, which is responsible for catalyzing the condensation of δ-aminolevulinic acid (ALA) to generate bilirubin (PBG). Its tertiary structure presents a typical TIM barrel folding (α/β barrel), and the active center is coordinated by cysteine and histidine residues to form zinc ions, ensuring catalytic efficiency. The stability of this enzyme depends on the binding of zinc ions. However, in lead poisoning, lead competitively displaces zinc, leading to the inhibition of enzyme activity and subsequently affecting the synthesis of hemoglobin.
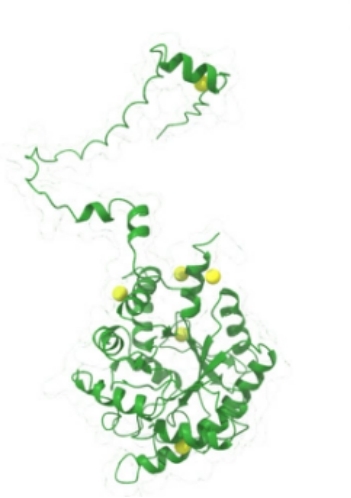 Fig. 1 Predicted protein structure and cysteine localization of ALAD1 in Arabidopsis thaliana.1
Fig. 1 Predicted protein structure and cysteine localization of ALAD1 in Arabidopsis thaliana.1
Key structural properties of ALAD:
- Octamer structure
- Zinc ion binding site
- Hydrophobic active pocket
- Key catalytic residues
- Lead-sensitive site
Functions of ALAD
The δ-aminolevulinic acid dehydrase (ALAD) encoded by the ALAD gene is a key enzyme in the heme biosynthesis pathway. Its function is not limited to catalytic reactions but also plays an important role in various physiological and pathological processes.
| Function | Description |
| Catalysis of heme synthesis | Catalyzing the condensation of two molecules of δ-aminolevulinic acid (ALA) to form bilirubin (PBG) is one of the rate-limiting steps in the synthesis of heme. |
| Heavy metal toxicity targets | Zinc ion binding sites are prone to competitive substitution by lead (Pb²⁺), leading to inhibition of enzyme activity and becoming a sensitive biomarker for lead poisoning. |
| Related to porphyria | Mutations in the ALAD gene can lead to rare ALAD-deficient porphyria, affecting hemoglobin metabolism and causing neurological and skin symptoms. |
| Regulation of oxidative stress | By maintaining heme homeostasis, it indirectly affects the activity of antioxidant enzymes (such as catalase) and participates in cell REDOX balance. |
| Energy metabolic support | Ensure the supply of hemoglobin, guarantee the normal operation of mitochondrial cytochrome, and maintain the efficiency of cellular energy (ATP) production. |
The enzymatic kinetic characteristics of ALAD exhibit typical metalloenzyme properties, with its activity strictly dependent on zinc ions, while the lead inhibitory effect shows a dose-dependent nature. This characteristic is widely applied in environmental toxicology assessment. This enzyme is highly expressed in hematopoietic tissues such as the liver and bone marrow. Its functional disorder will directly affect red blood cell production and oxygen transport capacity.
Applications of ALAD and ALAD Antibody in Literature
1. Ye, Qiang, et al. "Low expression of moonlight gene ALAD is correlated with poor prognosis in hepatocellular carcinoma." Gene 825 (2022): 146437. https://doi.org/10.1016/j.gene.2022.146437
This study constructed a prognostic model for HCC based on eight part-time genes, among which it was discovered for the first time that ALAD was lowly expressed in HCC and associated with a poor prognosis. The expression of ALAD decreases with pathological grade and can be used as a novel prognostic marker for HCC.
2. Perini, Jamila Alessandra, et al. "Genetic Polymorphism of Delta Aminolevulinic Acid Dehydratase (ALAD) Gene and Symptoms of Chronic Mercury Exposure in Munduruku Indigenous Children within the Brazilian Amazon." International Journal of Environmental Research and Public Health 18.16 (2021): 8746. https://doi.org/10.3390/ijerph18168746
This study investigated the impact of ALAD gene polymorphisms on chronic mercury exposure in indigenous children of the Amazon in Brazil. It was found that children carrying the ALAD CG genotype had higher blood mercury levels and presented with neurological symptoms such as visual impairment and memory loss, indicating that ALAD polymorphisms may prolong the mercury half-life and exacerbate neurotoxicity.
3. Yun, Li, Weixing Zhang, and Kejun Qin. "Relationship among maternal blood lead, ALAD gene polymorphism and neonatal neurobehavioral development." International Journal of Clinical and Experimental Pathology 8.6 (2015): 7277. https://europepmc.org/article/med/26261627
This study explores the relationship between maternal lead and ALAD gene polymorphisms and the neurobehavioral development of newborns. The results showed that the maternal blood lead level of the ALAD12 genotype was significantly higher than that of the ALAD11 genotype, and the neurobehavioral score of newborns in the high blood lead group was lower. The polymorphism of the ALAD gene indirectly affects the early neurodevelopment of newborns by influencing blood lead levels.
4. van Bemmel, Dana M., et al. "Comprehensive analysis of 5-aminolevulinic acid dehydrogenase (ALAD) variants and renal cell carcinoma risk among individuals exposed to lead." PloS one 6.7 (2011): e20432. https://doi.org/10.1371/journal.pone.0020432
This study indicates that ALAD gene polymorphisms (such as rs8177796 and rs2761016) may increase the risk of renal cell carcinoma and interact with occupational lead exposure. Research has found that carriers of specific ALAD variant genotypes have a significantly increased risk of cancer, suggesting that ALAD variants may promote the occurrence of renal cancer by influencing lead toxicity kinetics.
5. Miyaki, Koichi, et al. "Association between a polymorphism of aminolevulinate dehydrogenase (ALAD) gene and blood lead levels in Japanese subjects." International journal of environmental research and public health 6.3 (2009): 999-1009. https://doi.org/10.3390/ijerph6030999
A study in Japan found that non-occupational lead-exposed workers carrying the ALAD2 genotype had significantly higher blood lead levels than those carrying the ALAD1 genotype, but no differences in blood indicators such as hemoglobin were found. Studies have shown that ALAD gene polymorphisms affect blood lead concentrations under environmental lead exposure, suggesting that this genotype may increase the risk of lead poisoning.
Creative Biolabs: ALAD Antibodies for Research
Creative Biolabs specializes in the production of high-quality ALAD antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ALAD Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ALAD antibodies, custom preparations, or technical support, contact us at email.
Reference
- Wittmann, Daniel, Chao Wang, and Bernhard Grimm. "More indications for redox-sensitive cysteine residues of the Arabidopsis 5-aminolevulinate dehydratase." Frontiers in plant science 14 (2024): 1294802. https://doi.org/10.3389/fpls.2023.1294802
Anti-ALAD antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-COL1A2 Recombinant Antibody (CF108) (V2LY-1206-LY626)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-ESR1 Recombinant Antibody (Y31) (CBMAB-1208-YC)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-CD24 Recombinant Antibody (SN3) (CBMAB-C1037-CQ)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-ARHGAP5 Recombinant Antibody (54/P190-B) (CBMAB-P0070-YC)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Mouse Anti-APP Recombinant Antibody (DE2B4) (CBMAB-1122-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






