FTO Antibodies
Background
The FTO gene is located on human chromosome 16 and encodes a nucleic acid demethylase dependent on α -ketoglutarate. This enzyme affects the expression of downstream genes by regulating the modification levels such as N6-methyladenosine (m6A) in mRNA, and thereby participates in physiological processes such as energy metabolism, cell proliferation and differentiation. Extensive studies have shown that FTO gene polymorphisms are significantly associated with obesity risk, appetite regulation and weight gain, and the mechanism involves interference with the signaling pathways of the hypothalamic appetite center. Since its first confirmation of its association with obesity through genome-wide association analysis in 2007, FTO has become a key target in the study of metabolic diseases, providing an important model for understanding the role of epigenetic regulation in energy balance.
Structure of FTO
The protein encoded by the FTO gene is an α -ketoglutarate-dependent dioxygenase with a molecular weight of approximately 58 kDa. This molecular weight is relatively conserved among different species, but there are subtle variations caused by differences in amino acid sequences.
| Species | Human | Mouse | Rat | Zebrafish |
| Molecular Weight (kDa) | 58.3 | 58.1 | 58.2 | 57.8 |
| Primary Structural Differences | Highly conserved structure of catalytic domain | High homology with humans | High sequence similarity | The core functional domain is retained |
This protein is composed of approximately 500 amino acid residues, and its three-dimensional structure presents a typical double-stranded β -helical folding. The core of the protein structure is a catalytic center composed of the JmjC domain, which catalyzes the demethylation of the substrate through the synergy of an α -ketoglutaric acid binding site and an iron ion. Its secondary structure is mainly composed of β -folding and connecting rings, forming a hydrophobic cavity to accommodate nucleic acid substrates. The key aspartic acid residues are responsible for chelating iron ions, while the arginine residue network is crucial for recognizing and binding to methylated nucleic acids.
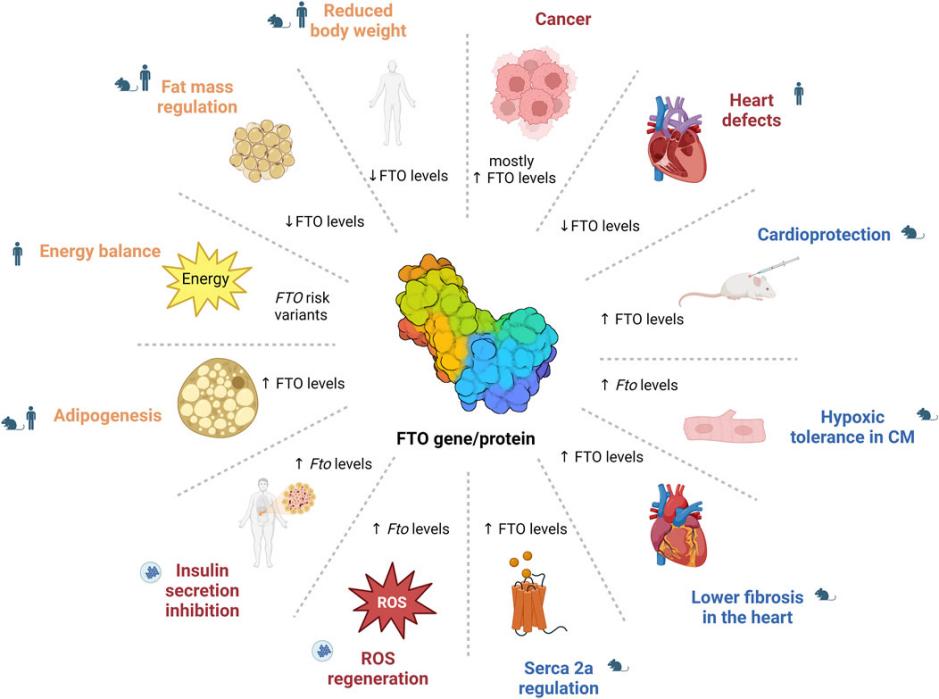 Fig. 1 The role of FTO in health and disease.1
Fig. 1 The role of FTO in health and disease.1
Key structural properties of FTO:
- Unique double-stranded β -helical folded conformation
- Hydrophobic core region surrounded by a catalytic activity center
- Demethylation function is achieved by relying on the active sites of Fe(II) and α -ketoglutaric acid
Functions of FTO
The core function of the FTO protein is to catalyze the demethylation of nucleic acid m6A to regulate gene expression. In addition, it is also involved in various physiological and pathological processes such as energy balance, cell differentiation and metabolic syndrome.
| Function | Description |
| m6A demethylation | As an α -ketoglutarate-dependent dioxygenase, it catalyzes the demethylation of N6-methyladenosine (m6A) in RNA and dynamically regulates the post-transcriptional expression level of target genes. |
| Regulation of energy metabolism | Demethylation affects appetite-related neural circuits and adipocyte differentiation in the hypothalamus, thus regulating the balance of energy intake, storage and consumption. |
| Susceptibility to obesity | Gene polymorphisms can significantly increase an individual's risk of obesity, and the mechanism is related to enhancing the expression of appetite-related genes (such as IRX3) and promoting lipid accumulation. |
| Cell differentiation participation | Participate in adipogenesis and osteogenesis differentiation process, the change of the enzyme activity ectomesenchymal stem cells directly affect the fate of the decision. |
| Maintenance of metabolic homeostasis | Through extensive transcriptomic-level regulation, it affects pathways such as glucose metabolism and insulin sensitivity, playing a significant role in maintaining overall metabolic homeostasis. |
The catalytic activity of FTO against m6A depends on Fe(II) and α -ketoglutarate. Its substrate range of action is not limited to mRNA but also includes certain types of snRNA, demonstrating its wide applicability in epigenetic regulation.
Applications of FTO and FTO Antibody in Literature
1. Azzam, Sarah Kassem, Habiba Alsafar, and Abdulrahim A. Sa**i. "FTO m6A demethylase in obesity and cancer: implications and underlying molecular mechanisms." International journal of molecular sciences 23.7 (2022): 3800. https://doi.org/10.3390/ijms23073800
The article indicates that FTO is the first discovered RNA m6A demethylase, and its gene polymorphism is associated with obesity and the risk of various cancers. This enzyme affects tumorigenesis and adipogenesis by regulating m6A modification, and its inhibitors have become a new strategy for targeted therapy. This article aims to explore the mechanism of action of FTO in diseases.
2. Benak, Daniel, et al. "FTO in health and disease." Frontiers in Cell and Developmental Biology 12 (2024): 1500394. https://doi.org/10.3389/fcell.2024.1500394
The article indicates that FTO is an important RNA demethylase that widely influences RNA metabolism. Its dysfunction is closely related to many diseases such as obesity, diabetes and cancer. Inhibitors targeting FTO have emerged as a highly promising new therapeutic strategy, opening up new avenues for the diagnosis and treatment of metabolic diseases and tumors.
3. Xu, Yawei, et al. "FTO-mediated autophagy promotes progression of clear cell renal cell carcinoma via regulating SIK2 mRNA stability." International Journal of Biological Sciences 18.15 (2022): 5943. https://doi.org/10.7150/ijbs.77774
This study reveals that FTO down-regulates the expression of SIK2 through the m6A-IGF2BP2 mechanism, inhibits autophagy, and thereby promotes the progression of clear cell renal cell carcinoma. Targeted inhibition of FTO can effectively curb tumor growth, indicating that FTO is a potential prognostic marker and therapeutic target for ccRCC.
4. Chang, Rui, et al. "Emerging roles of FTO in neuropsychiatric disorders." BioMed research international 2022.1 (2022): 2677312. https://doi.org/10.1155/2022/2677312
The article indicates that the FTO gene, as an m6A demethylase, is highly expressed in the central nervous system. It plays a significant role in the pathogenesis of neuropsychiatric disorders such as drug addiction, major depression and schizophrenia by regulating the m6A modification of related genes like dopamine.
5. Huang, Jiapeng, et al. "FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner." Journal of Experimental & Clinical Cancer Research 41.1 (2022): 42. https://doi.org/10.1186/s13046-022-02254-z
This study reveals for the first time the function of FTO as a tumor suppressor gene in PTC. It down-regulates APOE expression through m6A demethylation, thereby inhibiting the IL-6/JAK2/STAT3 signaling pathway and glycolysis, and ultimately curbing the growth of PTC tumors.
Creative Biolabs: FTO Antibodies for Research
Creative Biolabs specializes in the production of high-quality FTO antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom FTO Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our FTO antibodies, custom preparations, or technical support, contact us at email.
Reference
- Benak, Daniel, et al. "FTO in health and disease." Frontiers in Cell and Developmental Biology 12 (2024): 1500394. https://doi.org/10.3389/fcell.2024.1500394
Anti-FTO antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

-
Mouse Anti-BBS2 Recombinant Antibody (CBYY-0253) (CBMAB-0254-YY)

-
Mouse Anti-BCL2L1 Recombinant Antibody (H5) (CBMAB-1025CQ)

-
Mouse Anti-CHRNA9 Recombinant Antibody (8E4) (CBMAB-C9161-LY)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-FOSB Recombinant Antibody (CBXF-3593) (CBMAB-F2522-CQ)

-
Mouse Anti-CALR Recombinant Antibody (CBFYC-0763) (CBMAB-C0818-FY)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Mouse Anti-ARIH1 Recombinant Antibody (C-7) (CBMAB-A3563-YC)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Rat Anti-EPO Recombinant Antibody (16) (CBMAB-E1578-FY)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-ACE2 Recombinant Antibody (V2-179293) (CBMAB-A0566-YC)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






