GAPDH Antibodies
Background
The enzyme GAPDH serves purposes, in cells by participating in glycolysis. Can be found in different tissues within the body. It facilitates the transformation of glyceraldehyde‐three‐phosphate to 1,some linear text4 bisphosphoglycerate as part of its role in cellular energy production. Besides its metabolic duties GAPDH is also engaged in activities like DNA repair, determination of cell death and transportation of vesicles. Scientists first recognized this enzyme in the 1900s. Since then it has been extensively examined for its importance in essential cellular functions. Due to its structure and varied roles GAPDH is a focal point, for both research endeavors and diagnostic purposes.
Structure of GAPDH
GAPDH is a relatively small protein with a molecular weight of approximately 36 kDa. This weight may vary slightly between species due to differences in amino acid sequence and post-translational modifications.
| Species | Human | Mouse | Giant Panda | Medicago (Plant) | Arabidopsis thaliana |
| Molecular Weight (kDa) | 36 | 36 | 35.8 | 36 | 36 |
| Primary Structural Differences | Contains specific phosphorylation sites and is highly conserved across mammals | Similar to human GAPDH with high sequence homology | Contains specific glycosylation and phosphorylation sites, with a theoretical pI of 8.21 | Forms different oligomeric states (A2B2 and A4 tetramers) depending on the species | Forms different oligomeric states and is involved in the Calvin cycle |
The enzyme GAPDH contains approximately 330 amino acids and displays a compact structure through its primary sequence of alpha-helices and beta-sheets. The protein structure of GAPDH includes a central catalytic domain which enables efficient substrate binding and conversion. The enzyme's clear color in solution originates from its lack of prosthetic groups. GAPDH displays a secondary structure composed mainly of alpha-helices and beta-sheets named from A to G which form a hydrophilic active site to stabilize substrates. The active site residues form direct bonds with substrates and catalytic residues facilitate the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate.
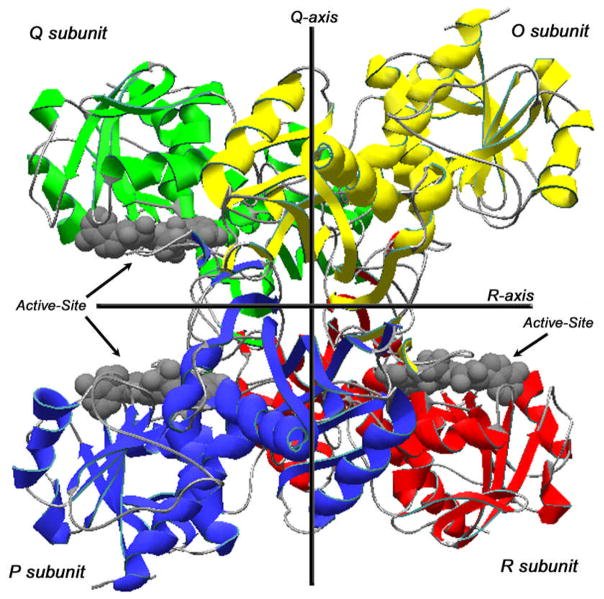 Fig. 1 GAPDH Structure.1
Fig. 1 GAPDH Structure.1
Key structural properties of GAPDH:
- Multifunctional enzyme in glycolysis and DNA repair
- Conserved structure across species
- Oligomeric states: forms tetramers and dimers
- Active sites for substrate binding and catalysis
- Post-translational modifications like phosphorylation
Functions of GAPDH
The main role of GAPDH is glycolysis and energy metabolism. However, it is also involved in various physiological processes, including DNA repair and apoptosis regulation.
| Function | Description |
| Glycolysis | Catalyzes the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate. |
| DNA Repair | Involved in base excision repair pathways. |
| Apoptosis Regulation | Participates in the regulation of programmed cell death. |
| Vesicle Transport | Assists in intracellular vesicle trafficking. |
| Non-Metabolic Roles | Functions in processes beyond glycolysis, such as stress response. |
The activity of GAPDH is allosterically regulated in contrast to many other glycolytic enzymes, indicating its sensitivity to cellular metabolic state and role in fine-tuning energy production.
Applications of GAPDH and GAPDH Antibody in Literature
1. Zhang, Jin-Ying, et al. "Critical protein GAPDH and its regulatory mechanisms in cancer cells." Cancer biology & medicine 12.1 (2015): 10-22. https://doi.org/ 10.7497/j.issn.2095-3941.2014.0019
This article delves into the protein GAPDH. How it is controlled in cancer cells discussing its impact, on cancer development and pointing out its promise as a target for treatment, in the field of oncology.
2. Colell, A., D. R. Green, and J. E. Ricci. "Novel roles for GAPDH in cell death and carcinogenesis." Cell Death & Differentiation 16.12 (2009): 1573-1581. https://doi.org/10.1038/cdd.2009.137
This article delves into functions of GAPDH, in cell death and the formation of tumors." It discusses how GAPDH plays a part, in apoptosis and the growth of tumors while underscoring its importance in the field of cancer research.
3. Nicholls, Craig, He Li, and Jun‐Ping Liu. "GAPDH: a common enzyme with uncommon functions." Clinical and Experimental Pharmacology and Physiology 39.8 (2012): 674-679. https://doi.org/10.1111/j.1440-1681.2011.05599.x
This article highlights GAPDH: a common enzyme with uncommon functions. It explores the diverse roles of GAPDH beyond glycolysis, including its involvement in apoptosis, DNA repair, and vesicle transport, emphasizing its multifunctionality in cellular processes.
4. Sirover, Michael A. "Pleiotropic effects of moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cancer progression, invasiveness, and metastases." Cancer and Metastasis Reviews 37 (2018): 665-676. https://doi.org/10.1007/s10555-018-9764-7
This article examines the pleiotropic effects of moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cancer progression, invasiveness, and metastasis. It explores how GAPDH contributes to multiple aspects of cancer biology beyond its metabolic role, highlighting its significance in tumor development and spread.
5. Liu, Shanshan, et al. "Glyceraldehyde‐3‐phosphate dehydrogenase promotes liver tumorigenesis by modulating phosphoglycerate dehydrogenase." Hepatology 66.2 (2017): 631-645. https://doi.org/10.1002/hep.29202
This article investigates how glyceraldehyde-3-phosphate dehydrogenase (GAPDH) promotes liver tumorigenesis by modulating phosphoglycerate dehydrogenase. It highlights the role of GAPDH in metabolic reprogramming and tumor progression, demonstrating its significance in liver cancer development.
Creative Biolabs: GAPDH Antibodies for Research
Creative Biolabs produces high-quality GAPDH antibodies for research and industrial applications. Our portfolio includes monoclonal and polyclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom GAPDH Antibody Solutions: Bespoke antibody development to fulfill precise research needs.
- Large-Scale Production: High-volume antibody manufacturing for industrial collaboration.
- Technical Guidance: Specialized advice for optimizing protocols and resolving technical challenges.
- Aliquoting Solutions: Pre-measured aliquots for stable long-term storage and reliable experimental results.
For further information on our GAPDH antibodies, custom services, or technical support, please contact us via info@creative-biolabs.com.
Reference
- Butterfield, D. Allan, Sarita S. Hardas, and Miranda L. Bader Lange. "Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration." Journal of Alzheimer's Disease 20.2 (2010): 369-393. https://doi.org/10.3233/JAD-2010-1375
Anti-GAPDH antibodies
 Loading...
Loading...
Hot products 
-
Rat Anti-FABP3 Recombinant Antibody (CBXF-2299) (CBMAB-F1612-CQ)

-
Mouse Anti-CIITA Recombinant Antibody (CBLC160-LY) (CBMAB-C10987-LY)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Mouse Anti-FOXA3 Recombinant Antibody (2A9) (CBMAB-0377-YC)

-
Mouse Anti-dsRNA Recombinant Antibody (2) (CBMAB-D1807-YC)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-AMACR Recombinant Antibody (CB34A) (CBMAB-CA034LY)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-APP Recombinant Antibody (DE2B4) (CBMAB-1122-CN)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Rat Anti-CD300A Recombinant Antibody (172224) (CBMAB-C0423-LY)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








