HSCB Antibodies
Background
The HSCB gene encodes a mitochondrial chaperone protein, which is mainly involved in the biosynthesis and transport of iron-sulfur clusters within vertebrate cells. This protein promotes the transfer of new iron-sulfur clusters to target proteins by interacting with iron-sulfur cluster assembly proteins. This process is crucial for maintaining cellular energy metabolism and mitochondrial function. During the process of red blood cell differentiation, HSCB particularly supports the establishment of the oxygen transport system by assisting in the maturation of enzymes related to heme synthesis. The molecular mechanism research of this gene made a breakthrough in the early 21st century. The three-dimensional structure analysis of it revealed the precise pattern of chaperone protein-receptor specific recognition, and the related results were included in the functional annotation database of the Human Genome Project. This highly specific molecular collaboration mechanism has become a classic model for the study of protein-protein interactions and provides a key molecular basis for understanding hereditary iron metabolism diseases, such as mitochondrial sideroblastic anemia.
Structure of HSCB
The molecular weight of the protein encoded by the HSCB gene is approximately 22 kDa, and its precise value varies slightly among different species due to sequence differences.
| Species | Human | Mouse | Zebrafish | Yeast |
| Molecular Weight (kDa) | 22.1 | 21.8 | 20.5 | 24.3 |
| Primary Structural Differences | The typical HSCB domain structure | The typical HSCB domain structure | Low homology and simplified function | Mitochondria contain additional targeted sequence |
The protein encoded by the HSCB gene is composed of 204 amino acids, and its spatial structure shows a characteristic "L" -shaped three-dimensional fold. The core function of this protein relies on a highly conserved iron-sulfur cluster binding domain, which specifically recognizes and binds to the receptor protein through the thiol group of the cysteine residue. Its secondary structure is mainly composed of 9 β -folds and 3 α -helices, which jointly enclose a hydrophobic substrate-binding pocket. It is particularly worth noting that the arginine residue at position 138 stabilizes its spatial conformation through coordination with the iron-sulfur cluster, while the tyrosine at position 97 precisely regulates its interaction interface with the companion protein HSC20 through π-π stacking. This sophisticated molecular mechanism ensures the efficient transport and precise assembly of the iron-sulfur cluster within the cell.
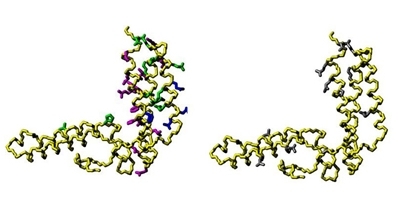 Fig. 1 Mapping the interaction surface on HscB (PDB code: 1FPO).1
Fig. 1 Mapping the interaction surface on HscB (PDB code: 1FPO).1
Key structural properties of HSCB:
- Unique "L" -shaped three-dimensional folding configuration
- Hydrophobic core forming the substrate combination of pocket
- Highly conserved iron-sulfur cluster cooperative binding interface
- Arginine-tyrosine molecular switch regulates chaperone protein recognition
Functions of HSCB
The core function of the HSCB gene-encoded protein is to act as a mitochondrial chaperone to mediate the transport and assembly of iron-sulfur clusters. However, it is also widely involved in various cellular processes, including mitochondrial protein quality control, reactive oxygen species balance and cell cycle regulation.
| Function | Description |
| Iron-sulfur cluster transport | Specifically recognize and bind to neogenic iron-sulfur clusters, and deliver them to target proteins such as dehydrogenase and respiratory chain complexes through conformational changes. |
| Mitochondrial biogenesis | Assist in the maturation of key enzymes such as cT-aconitase and complex I, and directly maintain the functionality of the tricarboxylic acid cycle and oxidative phosphorylation. |
| Maintenance of genomic stability | By ensuring the iron-sulfur cluster assembly of DNA repair enzymes (such as XPD helicase), it supports the accurate replication and damage repair of nuclear DNA. |
| Regulation of red blood cell maturation | It promotes the activation of heme synthase during hematopoiesis, and its functional deficiency can lead to mitochondrial sideroblastic anemia. |
| Oxidative stress buffer | By precisely regulating the insertion of iron-sulfur clusters, the Fenton reaction triggered by free iron is reduced, thereby alleviating the oxidative stress within mitochondria. |
The HSCB protein exhibits a highly specific linear binding pattern for substrate recognition, which contrasts sharply with the broad-spectrum binding characteristics of universal molecular chaperones, revealing its functional positioning as a precise "assemper" rather than a "universal assistant" in the biosynthesis of iron-sulfur clusters.
Applications of HSCB and HSCB Antibody in Literature
1. Kim J H, Alderson T R, et al. "Nucleotide-dependent interactions within a specialized Hsp70/Hsp40 complex involved in Fe–S cluster biogenesis." Journal of the American Chemical Society 136.33 (2014): 11586-11589. https://doi.org/10.1021/ja5055252
This study revealed through nuclear magnetic resonance technology that the cochaperone protein HscB of Escherichia coli interacts directly with ATP-bound HscA through the "HPD" motif in its J domain. Meanwhile, the C-terminal region of HscB also has a secondary binding site that is not strictly dependent on nucleotides, jointly regulating the transfer process of iron-sulfur clusters.
2. Puglisi, Rita, et al. "A New Tessera into the interactome of the isc operon: a novel interaction between HscB and IscS." Frontiers in Molecular Biosciences 3 (2016): 48. https://doi.org/10.3389/fmolb.2016.00048
New research has found that there is a weak interaction between the cochaperone protein HscB of Escherichia coli and the desulfurizer enzyme IscS. This region of action is located on the long stem of the HscB molecule and overlaps with the surface of other proteins bound to IscS. This indicates that HscB may be a component of the IscS complex, providing a brand-new perspective for understanding its function.
3. Liu, Gang, et al. "PI3K/HSCB axis facilitates FOG1 nuclear translocation to promote erythropoiesis and megakaryopoiesis." Elife 13 (2024): RP95815. https://doi.org/10.7554/eLife.95815
Research has found that HSCB has a new function in the differentiation of erythroid and megakaryocytic systems that is independent of iron-sulfur cluster delivery. After HSCB is phosphorylated by PI3K, it can mediate the degradation of TACC3 protein, which retains the FOG1 transcription factor, thereby promoting the nuclear entry of FOG1, which is crucial for blood cell production.
4. Bitto, Eduard, et al. "Structure of human J-type co-chaperone HscB reveals a tetracysteine metal-binding domain." Journal of Biological Chemistry 283.44 (2008): 30184-30192. https://doi.org/10.1074/jbc.M804746200
The article indicates that the human mitochondrial cochaperone protein HscB is a J protein, whose structure is similar to that of the Escherichia coli homolog, but it has an additional metal-binding domain at the N-terminal. During the maturation process of iron-sulfur cluster proteins, HscB collaborates with the companion protein HscA, jointly promoting the efficient transfer of iron-sulfur clusters from IscU to the target protein by delivering the scaffold protein IscU to HscA and enhancing its ATPase activity.
5. Kim, Jin Hae, et al. "Specialized Hsp70 chaperone (HscA) binds preferentially to the disordered form, whereas J-protein (HscB) binds preferentially to the structured form of the iron-sulfur cluster scaffold protein (IscU)." Journal of Biological Chemistry 287.37 (2012): 31406-31413. https://doi.org/10.1074/jbc.M112.352617
The article indicates that HscB is a J protein, which preferentially binds to the structured conformation (S state) of IscU and promotes the conformational equilibrium of IscU to shift towards the S state. Studies have shown that the interaction between HscB and IscU plays a significant role in the assembly and transfer of iron-sulfur clusters.
Creative Biolabs: HSCB Antibodies for Research
Creative Biolabs specializes in the production of high-quality HSCB antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom HSCB Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our HSCB antibodies, custom preparations, or technical support, contact us at email.
Reference
- Han, Jun, et al. "Effect of changes in the structure of myoglobin on the color of meat products." Food Materials Research 4.1 (2024). https://doi.org/10.48130/fmr-0024-0003
Anti-HSCB antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Mouse Anti-CEMIP Recombinant Antibody (3C12) (CBMAB-K0296-LY)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Rat Anti-FABP3 Recombinant Antibody (CBXF-2299) (CBMAB-F1612-CQ)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0435) (CBMAB-0437-YY)

-
Mouse Anti-FLT1 Recombinant Antibody (11) (CBMAB-V0154-LY)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-FeLV g27 Recombinant Antibody (1) (CBMAB-V208-1714-FY)

-
Rat Anti-AChR Recombinant Antibody (V2-12500) (CBMAB-0990-CN)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






